+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6jpa | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Rabbit Cav1.1-Verapamil Complex | |||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / Membrane protein complex modulated by FDA approved drug. | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationhigh voltage-gated calcium channel activity / L-type voltage-gated calcium channel complex / positive regulation of muscle contraction / regulation of release of sequestered calcium ion into cytosol / regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / cellular response to caffeine / calcium ion import across plasma membrane / voltage-gated calcium channel activity / release of sequestered calcium ion into cytosol / T-tubule ...high voltage-gated calcium channel activity / L-type voltage-gated calcium channel complex / positive regulation of muscle contraction / regulation of release of sequestered calcium ion into cytosol / regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / cellular response to caffeine / calcium ion import across plasma membrane / voltage-gated calcium channel activity / release of sequestered calcium ion into cytosol / T-tubule / muscle contraction / calcium channel regulator activity / sarcolemma / calcium ion transmembrane transport / transmembrane transporter binding / calmodulin binding / metal ion binding / plasma membrane Similarity search - Function | |||||||||||||||||||||
| Biological species |  | |||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.6 Å | |||||||||||||||||||||
 Authors Authors | Zhao, Y. / Huang, G. / Wu, J. / Yan, N. | |||||||||||||||||||||
| Funding support |  China, 6items China, 6items
| |||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2019 Journal: Cell / Year: 2019Title: Molecular Basis for Ligand Modulation of a Mammalian Voltage-Gated Ca Channel. Authors: Yanyu Zhao / Gaoxingyu Huang / Jianping Wu / Qiurong Wu / Shuai Gao / Zhen Yan / Jianlin Lei / Nieng Yan /   Abstract: The L-type voltage-gated Ca (Ca) channels are modulated by various compounds exemplified by 1,4-dihydropyridines (DHP), benzothiazepines (BTZ), and phenylalkylamines (PAA), many of which have been ...The L-type voltage-gated Ca (Ca) channels are modulated by various compounds exemplified by 1,4-dihydropyridines (DHP), benzothiazepines (BTZ), and phenylalkylamines (PAA), many of which have been used for characterizing channel properties and for treatment of hypertension and other disorders. Here, we report the cryoelectron microscopy (cryo-EM) structures of Ca1.1 in complex with archetypal antagonistic drugs, nifedipine, diltiazem, and verapamil, at resolutions of 2.9 Å, 3.0 Å, and 2.7 Å, respectively, and with a DHP agonist Bay K 8644 at 2.8 Å. Diltiazem and verapamil traverse the central cavity of the pore domain, directly blocking ion permeation. Although nifedipine and Bay K 8644 occupy the same fenestration site at the interface of repeats III and IV, the coordination details support previous functional observations that Bay K 8644 is less favored in the inactivated state. These structures elucidate the modes of action of different Ca ligands and establish a framework for structure-guided drug discovery. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6jpa.cif.gz 6jpa.cif.gz | 533.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6jpa.ent.gz pdb6jpa.ent.gz | 409.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6jpa.json.gz 6jpa.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jp/6jpa https://data.pdbj.org/pub/pdb/validation_reports/jp/6jpa ftp://data.pdbj.org/pub/pdb/validation_reports/jp/6jpa ftp://data.pdbj.org/pub/pdb/validation_reports/jp/6jpa | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  9868MC  9866C  9867C  9869C  6jp5C  6jp8C  6jpbC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 172146.531 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Voltage-dependent calcium channel ... , 2 types, 2 molecules EF
| #2: Protein | Mass: 25082.254 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #5: Protein | Mass: 118641.398 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Voltage-dependent L-type calcium channel subunit beta- ... , 2 types, 2 molecules BC
| #3: Protein | Mass: 49642.430 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #4: Protein | Mass: 57898.285 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Sugars , 4 types, 15 molecules 
| #6: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-3)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #7: Polysaccharide | Source method: isolated from a genetically manipulated source #8: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-3)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-3)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #9: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 6 types, 17 molecules 



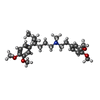
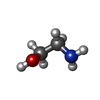





| #10: Chemical | | #11: Chemical | ChemComp-3PE / #12: Chemical | #13: Chemical | ChemComp-9Z9 / ( | #14: Chemical | #15: Chemical | ChemComp-ETA / | |
|---|
-Details
| Has protein modification | Y |
|---|---|
| Sequence details | As for the unusual connection between Y619-S621 of Chain F, the original amino acid for S620 is ...As for the unusual connection between Y619-S621 of Chain F, the original amino acid for S620 is Y620 and the density for this large side chain is missing from reconstruction. Authors suspect this to be a result of alternative splicing. The density in this beta strand, Y619 and S621, is very clear and allows accurate side chain assignment for this area and surrounding areas. |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Units: KILODALTONS/NANOMETER / Experimental value: NO | ||||||||||||||||||
| Source (natural) | Organism:  | ||||||||||||||||||
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 48 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 QUANTUM (4k x 4k) |
| EM imaging optics | Energyfilter name: GIF Quantum LS / Energyfilter slit width: 20 eV |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING ONLY |
|---|---|
| 3D reconstruction | Resolution: 2.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 433477 / Symmetry type: POINT |
 Movie
Movie Controller
Controller











 PDBj
PDBj













