Entry Database : PDB / ID : 6greTitle Crystal structure of the tandem DUF26 ectodomain from the Arabidopsis thaliana cysteine-rich receptor-like protein PDLP5. Cysteine-rich repeat secretory protein 2 Keywords / / / / / / Function / homology Function Domain/homology Component
Biological species Arabidopsis thaliana (thale cress)Method / / / Resolution : 1.29 Å Authors Brandt, B. / Hothorn, M. Funding support Organization Grant number Country Swiss National Science Foundation 31003A_176237
Journal : Commun Biol / Year : 2019Title : Mechanistic insights into the evolution of DUF26-containing proteins in land plants.Authors : Vaattovaara, A. / Brandt, B. / Rajaraman, S. / Safronov, O. / Veidenberg, A. / Luklova, M. / Kangasjarvi, J. / Loytynoja, A. / Hothorn, M. / Salojarvi, J. / Wrzaczek, M. History Deposition Jun 11, 2018 Deposition site / Processing site Revision 1.0 Dec 26, 2018 Provider / Type Revision 1.1 Feb 27, 2019 Group / Database references / Category / citation_author / pdbx_database_procItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year Revision 2.0 Jul 29, 2020 Group Atomic model / Data collection ... Atomic model / Data collection / Derived calculations / Structure summary Category atom_site / chem_comp ... atom_site / chem_comp / entity / pdbx_branch_scheme / pdbx_chem_comp_identifier / pdbx_entity_branch / pdbx_entity_branch_descriptor / pdbx_entity_branch_link / pdbx_entity_branch_list / pdbx_entity_nonpoly / pdbx_nonpoly_scheme / pdbx_struct_assembly_gen / pdbx_struct_conn_angle / struct_asym / struct_conn / struct_site / struct_site_gen Item _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.type_symbol / _chem_comp.name / _chem_comp.type / _pdbx_entity_nonpoly.entity_id / _pdbx_entity_nonpoly.name / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.pdbx_role / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id Description / Provider / Type Revision 2.1 Nov 6, 2024 Group / Database references / Structure summaryCategory chem_comp / chem_comp_atom ... chem_comp / chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature Item / _database_2.pdbx_DOI / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  SAD / Resolution: 1.29 Å
SAD / Resolution: 1.29 Å  Authors
Authors Switzerland, 1items
Switzerland, 1items  Citation
Citation Journal: Commun Biol / Year: 2019
Journal: Commun Biol / Year: 2019 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6gre.cif.gz
6gre.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6gre.ent.gz
pdb6gre.ent.gz PDB format
PDB format 6gre.json.gz
6gre.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/gr/6gre
https://data.pdbj.org/pub/pdb/validation_reports/gr/6gre ftp://data.pdbj.org/pub/pdb/validation_reports/gr/6gre
ftp://data.pdbj.org/pub/pdb/validation_reports/gr/6gre Links
Links Assembly
Assembly

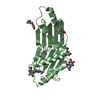
 Components
Components
 Trichoplusia ni (cabbage looper) / Strain (production host): Tnao / References: UniProt: Q8GUJ2
Trichoplusia ni (cabbage looper) / Strain (production host): Tnao / References: UniProt: Q8GUJ2










 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SLS
SLS  / Beamline: X06DA / Wavelength: 1.000033 Å
/ Beamline: X06DA / Wavelength: 1.000033 Å Processing
Processing SAD / Resolution: 1.29→37.77 Å / Cor.coef. Fo:Fc: 0.972 / Cor.coef. Fo:Fc free: 0.964 / SU B: 1.206 / SU ML: 0.047 / Cross valid method: THROUGHOUT / ESU R: 0.051 / ESU R Free: 0.053 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
SAD / Resolution: 1.29→37.77 Å / Cor.coef. Fo:Fc: 0.972 / Cor.coef. Fo:Fc free: 0.964 / SU B: 1.206 / SU ML: 0.047 / Cross valid method: THROUGHOUT / ESU R: 0.051 / ESU R Free: 0.053 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS Movie
Movie Controller
Controller













 PDBj
PDBj




