+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6.0E+76 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of Human Transthyretin Asp38Ala/Thr119Met Mutant | ||||||
 Components Components | Transthyretin | ||||||
 Keywords Keywords | TRANSPORT PROTEIN / human transthyretin / amyloid / transthyretin / mutant | ||||||
| Function / homology |  Function and homology information Function and homology informationDefective visual phototransduction due to STRA6 loss of function / negative regulation of glomerular filtration / The canonical retinoid cycle in rods (twilight vision) / purine nucleobase metabolic process / hormone binding / Non-integrin membrane-ECM interactions / molecular sequestering activity / phototransduction, visible light / retinoid metabolic process / Retinoid metabolism and transport ...Defective visual phototransduction due to STRA6 loss of function / negative regulation of glomerular filtration / The canonical retinoid cycle in rods (twilight vision) / purine nucleobase metabolic process / hormone binding / Non-integrin membrane-ECM interactions / molecular sequestering activity / phototransduction, visible light / retinoid metabolic process / Retinoid metabolism and transport / hormone activity / azurophil granule lumen / Amyloid fiber formation / Neutrophil degranulation / protein-containing complex binding / protein-containing complex / extracellular space / extracellular exosome / extracellular region / identical protein binding Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.6 Å SYNCHROTRON / Resolution: 1.6 Å | ||||||
 Authors Authors | Saelices, L. / Chung, K. / Sawaya, M.R. / Cascio, D. / Eisenberg, D. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structural Variants of Transthyretin Authors: Saelices, L. / Chung, K. / Esswein, S. / Chou, J. / Liang, W. / Li, J.H. / Sawaya, M.R. / Cascio, D. / Eisenberg, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6e76.cif.gz 6e76.cif.gz | 107 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6e76.ent.gz pdb6e76.ent.gz | 81.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6e76.json.gz 6e76.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e7/6e76 https://data.pdbj.org/pub/pdb/validation_reports/e7/6e76 ftp://data.pdbj.org/pub/pdb/validation_reports/e7/6e76 ftp://data.pdbj.org/pub/pdb/validation_reports/e7/6e76 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6e6zC  6e70C  6e71C  6e72C  6e73C  6e74C  6e75C  6e77C  6e78C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 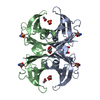
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: 1
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj



