+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 6avr | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Human alpha-V beta-3 Integrin (intermediate conformation) in complex with the therapeutic antibody LM609 | ||||||
 要素 要素 |
| ||||||
 キーワード キーワード | SIGNALING PROTEIN / alpha-V beta-3 integrin / LM609 / vitaxin / abegrin | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報integrin alphav-beta8 complex / integrin alphav-beta6 complex / transforming growth factor beta production / negative regulation of entry of bacterium into host cell / integrin alphav-beta5 complex / opsonin binding / integrin alphav-beta1 complex / Cross-presentation of particulate exogenous antigens (phagosomes) / extracellular matrix protein binding / tube development ...integrin alphav-beta8 complex / integrin alphav-beta6 complex / transforming growth factor beta production / negative regulation of entry of bacterium into host cell / integrin alphav-beta5 complex / opsonin binding / integrin alphav-beta1 complex / Cross-presentation of particulate exogenous antigens (phagosomes) / extracellular matrix protein binding / tube development / regulation of serotonin uptake / positive regulation of adenylate cyclase-inhibiting opioid receptor signaling pathway / alpha9-beta1 integrin-ADAM8 complex / regulation of trophoblast cell migration / integrin alphaIIb-beta3 complex / regulation of postsynaptic neurotransmitter receptor diffusion trapping / maintenance of postsynaptic specialization structure / alphav-beta3 integrin-vitronectin complex / regulation of extracellular matrix organization / positive regulation of glomerular mesangial cell proliferation / Laminin interactions / platelet alpha granule membrane / integrin alphav-beta3 complex / negative regulation of lipoprotein metabolic process / entry into host cell by a symbiont-containing vacuole / alphav-beta3 integrin-PKCalpha complex / fibrinogen binding / blood coagulation, fibrin clot formation / alphav-beta3 integrin-HMGB1 complex / vascular endothelial growth factor receptor 2 binding / negative regulation of lipid transport / regulation of phagocytosis / positive regulation of vascular endothelial growth factor signaling pathway / regulation of release of sequestered calcium ion into cytosol / Elastic fibre formation / mesodermal cell differentiation / cell-substrate junction assembly / alphav-beta3 integrin-IGF-1-IGF1R complex / positive regulation of bone resorption / glycinergic synapse / platelet-derived growth factor receptor binding / transforming growth factor beta binding / positive regulation of small GTPase mediated signal transduction / filopodium membrane / extracellular matrix binding / angiogenesis involved in wound healing / wound healing, spreading of epidermal cells / positive regulation of vascular endothelial growth factor receptor signaling pathway / apolipoprotein A-I-mediated signaling pathway / positive regulation of cell adhesion mediated by integrin / regulation of bone resorption / negative regulation of low-density lipoprotein particle clearance / apoptotic cell clearance / positive regulation of fibroblast migration / integrin complex / positive regulation of smooth muscle cell migration / heterotypic cell-cell adhesion / smooth muscle cell migration / Molecules associated with elastic fibres / cell adhesion mediated by integrin / negative chemotaxis / positive regulation of cell-matrix adhesion / Mechanical load activates signaling by PIEZO1 and integrins in osteocytes / Syndecan interactions / positive regulation of osteoblast proliferation / p130Cas linkage to MAPK signaling for integrins / cellular response to insulin-like growth factor stimulus / protein disulfide isomerase activity / regulation of postsynaptic neurotransmitter receptor internalization / microvillus membrane / cell-substrate adhesion / platelet-derived growth factor receptor signaling pathway / endodermal cell differentiation / PECAM1 interactions / GRB2:SOS provides linkage to MAPK signaling for Integrins / TGF-beta receptor signaling activates SMADs / fibronectin binding / positive regulation of intracellular signal transduction / lamellipodium membrane / negative regulation of macrophage derived foam cell differentiation / negative regulation of lipid storage / ECM proteoglycans / Integrin cell surface interactions / negative regulation of endothelial cell apoptotic process / positive regulation of T cell migration / vasculogenesis / specific granule membrane / voltage-gated calcium channel activity / coreceptor activity / cell adhesion molecule binding / cellular response to platelet-derived growth factor stimulus / phagocytic vesicle / ERK1 and ERK2 cascade / positive regulation of endothelial cell proliferation / Integrin signaling / extrinsic apoptotic signaling pathway in absence of ligand / positive regulation of substrate adhesion-dependent cell spreading / positive regulation of smooth muscle cell proliferation / positive regulation of endothelial cell migration / substrate adhesion-dependent cell spreading 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
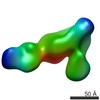
| 手法 | 電子顕微鏡法 / 単粒子再構成法 / ネガティブ染色法 / 解像度: 35 Å | ||||||
 データ登録者 データ登録者 | Borst, A.J. / James, Z.N. / Zagotta, W.N. / Ginsberg, M. / Rey, F.A. / DiMaio, F. / Backovic, M. / Veesler, D. | ||||||
 引用 引用 |  ジャーナル: Structure / 年: 2017 ジャーナル: Structure / 年: 2017タイトル: The Therapeutic Antibody LM609 Selectively Inhibits Ligand Binding to Human αβ Integrin via Steric Hindrance. 著者: Andrew J Borst / Zachary M James / William N Zagotta / Mark Ginsberg / Felix A Rey / Frank DiMaio / Marija Backovic / David Veesler /   要旨: The LM609 antibody specifically recognizes αβ integrin and inhibits angiogenesis, bone resorption, and viral infections in an arginine-glycine-aspartate-independent manner. LM609 entered phase II ...The LM609 antibody specifically recognizes αβ integrin and inhibits angiogenesis, bone resorption, and viral infections in an arginine-glycine-aspartate-independent manner. LM609 entered phase II clinical trials for the treatment of several cancers and was also used for αβ-targeted radioimmunotherapy. To elucidate the mechanisms of recognition and inhibition of αβ integrin, we solved the structure of the LM609 antigen-binding fragment by X-ray crystallography and determined its binding affinity for αβ. Using single-particle electron microscopy, we show that LM609 binds at the interface between the β-propeller domain of the α chain and the βI domain of the β chain, near the RGD-binding site, of all observed integrin conformational states. Integrating these data with fluorescence size-exclusion chromatography, we demonstrate that LM609 sterically hinders access of large ligands to the RGD-binding pocket, without obstructing it. This work provides a structural framework to expedite future efforts utilizing LM609 as a diagnostic or therapeutic tool. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  6avr.cif.gz 6avr.cif.gz | 307.3 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb6avr.ent.gz pdb6avr.ent.gz | 209.9 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  6avr.json.gz 6avr.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/av/6avr https://data.pdbj.org/pub/pdb/validation_reports/av/6avr ftp://data.pdbj.org/pub/pdb/validation_reports/av/6avr ftp://data.pdbj.org/pub/pdb/validation_reports/av/6avr | HTTPS FTP |
|---|
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
|
|---|---|
| 1 |
|
- 要素
要素
| #1: タンパク質 | 分子量: 105894.188 Da / 分子数: 1 / 断片: UNP residues 31-987 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: ITGAV, MSK8, VNRA, VTNR Homo sapiens (ヒト) / 遺伝子: ITGAV, MSK8, VNRA, VTNR発現宿主:  参照: UniProt: P06756 |
|---|---|
| #2: タンパク質 | 分子量: 76523.125 Da / 分子数: 1 / 断片: UNP residues 27-718 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: ITGB3, GP3A Homo sapiens (ヒト) / 遺伝子: ITGB3, GP3A発現宿主:  参照: UniProt: P05106 |
| #3: 抗体 | 分子量: 27223.100 Da / 分子数: 1 / 由来タイプ: 組換発現 / 由来: (組換発現)  発現宿主:  |
| #4: 抗体 | 分子量: 23628.977 Da / 分子数: 1 / 由来タイプ: 組換発現 / 由来: (組換発現)  発現宿主:  |
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: PARTICLE / 3次元再構成法: 単粒子再構成法 |
- 試料調製
試料調製
| 構成要素 | 名称: Quaternary complex of human alpha-V beta-3 integrin with the Fab LM609 タイプ: COMPLEX / Entity ID: all / 由来: RECOMBINANT |
|---|---|
| 分子量 | 値: 0.23 MDa / 実験値: NO |
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 由来(組換発現) | 生物種:  |
| 緩衝液 | pH: 8 |
| 試料 | 包埋: NO / シャドウイング: NO / 染色: YES / 凍結: NO |
| 染色 | タイプ: NEGATIVE / 染色剤: Uranyl formate |
| 試料支持 | グリッドの材料: COPPER / グリッドのサイズ: 400 divisions/in. / グリッドのタイプ: C-flat 2/0.5 |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 顕微鏡 | モデル: FEI TECNAI 12 |
|---|---|
| 電子銃 | 電子線源: LAB6 / 加速電圧: 120 kV / 照射モード: FLOOD BEAM |
| 電子レンズ | モード: BRIGHT FIELD |
| 撮影 | 電子線照射量: 30 e/Å2 フィルム・検出器のモデル: GATAN ULTRASCAN 4000 (4k x 4k) |
- 解析
解析
| EMソフトウェア |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTF補正 | タイプ: NONE | |||||||||
| 3次元再構成 | 解像度: 35 Å / 解像度の算出法: FSC 0.5 CUT-OFF / 粒子像の数: 650 / 対称性のタイプ: POINT | |||||||||
| 原子モデル構築 | プロトコル: FLEXIBLE FIT / 空間: REAL |
 ムービー
ムービー コントローラー
コントローラー



 UCSF Chimera
UCSF Chimera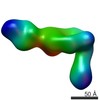












 PDBj
PDBj













