[English] 日本語
 Yorodumi
Yorodumi- PDB-6aq4: CRYSTAL STRUCTURE OF PROTEIN CiTE FROM MYCOBACTERIUM TUBERCULOSIS... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6aq4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF PROTEIN CiTE FROM MYCOBACTERIUM TUBERCULOSIS IN COMPLEX WITH MAGNESIUM, PYRUVATE AND CITRAMALYL-COA | |||||||||
 Components Components | Citrate lyase subunit beta-like protein | |||||||||
 Keywords Keywords | LYASE / Mycobacterium tuberculosis / CitE / Citramalyl-CoA / TIM-barrel fold / trimer | |||||||||
| Function / homology |  Function and homology information Function and homology informationLyases; Carbon-carbon lyases / oxaloacetate metabolic process / lyase activity / magnesium ion binding Similarity search - Function | |||||||||
| Biological species |  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.825 Å MOLECULAR REPLACEMENT / Resolution: 1.825 Å | |||||||||
 Authors Authors | Fedorov, A.A. / Fedorov, E.V. / Wang, H. / Bonanno, J.B. / Carvalho, L. / Almo, S.C. | |||||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2019 Journal: Proc.Natl.Acad.Sci.USA / Year: 2019Title: An essential bifunctional enzyme inMycobacterium tuberculosisfor itaconate dissimilation and leucine catabolism. Authors: Wang, H. / Fedorov, A.A. / Fedorov, E.V. / Hunt, D.M. / Rodgers, A. / Douglas, H.L. / Garza-Garcia, A. / Bonanno, J.B. / Almo, S.C. / de Carvalho, L.P.S. #1:  Journal: Biorxiv / Year: 2018 Journal: Biorxiv / Year: 2018Title: Discovery of a novel stereospecific beta-hydroxyacyl-CoA lyase/thioesterase shared by three metabolic pathways in Mycobacterium tuberculosis Authors: Wang, H. / Fedorov, A.A. / Fedorov, E.V. / Hunt, D.M. / Rodgers, A. / Garza-Garcia, A. / Bonanno, J.B. / Almo, S.C. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6aq4.cif.gz 6aq4.cif.gz | 329.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6aq4.ent.gz pdb6aq4.ent.gz | 268 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6aq4.json.gz 6aq4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aq/6aq4 https://data.pdbj.org/pub/pdb/validation_reports/aq/6aq4 ftp://data.pdbj.org/pub/pdb/validation_reports/aq/6aq4 ftp://data.pdbj.org/pub/pdb/validation_reports/aq/6aq4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6arbC  6as5C  6chuC  6cj3C  6cj4C  1u5hS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
| ||||||||
| Details | trimer by gel filtration |
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 29967.939 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria)Gene: citE, Rv2498c / Production host:  |
|---|
-Non-polymers , 7 types, 489 molecules 
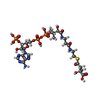











| #2: Chemical | | #3: Chemical | #4: Chemical | #5: Chemical | #6: Chemical | ChemComp-CL / #7: Chemical | ChemComp-PYR / | #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.62 Å3/Da / Density % sol: 53.1 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 6.8 / Details: 0.4M Ammonium phosphate, no buffer |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 31-ID / Wavelength: 0.97931 Å / Beamline: 31-ID / Wavelength: 0.97931 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jul 11, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97931 Å / Relative weight: 1 |
| Reflection | Resolution: 1.825→29.276 Å / Num. obs: 154090 / % possible obs: 94.8 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 4.2 % / Rmerge(I) obs: 0.052 / Net I/σ(I): 17.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1U5H Resolution: 1.825→29.276 Å / SU ML: 0.58 / Cross valid method: FREE R-VALUE / σ(F): 0.31 / Phase error: 25.88 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 1.06 Å / VDW probe radii: 1.3 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 43.627 Å2 / ksol: 0.34 e/Å3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.825→29.276 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj








