[English] 日本語
 Yorodumi
Yorodumi- PDB-5xr1: Structure of FLN IG21 domain in complex with C-terminal peptide o... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5xr1 | ||||||
|---|---|---|---|---|---|---|---|
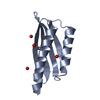
| Title | Structure of FLN IG21 domain in complex with C-terminal peptide of beta-2 | ||||||
 Components Components | C-terminal peptide of Integrin beta-2,Filamin-A IG21 domain | ||||||
 Keywords Keywords | SIGNALING PROTEIN / Integrin / cytosolic tail | ||||||
| Function / homology |  Function and homology information Function and homology informationintegrin alphaX-beta2 complex / regulation of membrane repolarization during atrial cardiac muscle cell action potential / regulation of membrane repolarization during cardiac muscle cell action potential / positive regulation of neutrophil degranulation / cellular extravasation / establishment of Sertoli cell barrier / integrin alphaM-beta2 complex / Myb complex / integrin alphaL-beta2 complex / glycoprotein Ib-IX-V complex ...integrin alphaX-beta2 complex / regulation of membrane repolarization during atrial cardiac muscle cell action potential / regulation of membrane repolarization during cardiac muscle cell action potential / positive regulation of neutrophil degranulation / cellular extravasation / establishment of Sertoli cell barrier / integrin alphaM-beta2 complex / Myb complex / integrin alphaL-beta2 complex / glycoprotein Ib-IX-V complex / ICAM-3 receptor activity / adenylate cyclase-inhibiting dopamine receptor signaling pathway / formation of radial glial scaffolds / positive regulation of integrin-mediated signaling pathway / actin crosslink formation / blood coagulation, intrinsic pathway / : / tubulin deacetylation / complement component C3b binding / OAS antiviral response / Toll Like Receptor 4 (TLR4) Cascade / positive regulation of actin filament bundle assembly / positive regulation of neuron migration / leukocyte migration involved in inflammatory response / neutrophil migration / protein localization to bicellular tight junction / Fc-gamma receptor I complex binding / Cell-extracellular matrix interactions / apical dendrite / positive regulation of potassium ion transmembrane transport / positive regulation of platelet activation / positive regulation of neural precursor cell proliferation / protein localization to cell surface / wound healing, spreading of cells / positive regulation of leukocyte adhesion to vascular endothelial cell / integrin complex / podosome / negative regulation of transcription by RNA polymerase I / heterotypic cell-cell adhesion / regulation of peptidyl-tyrosine phosphorylation / cell adhesion mediated by integrin / megakaryocyte development / phagocytosis, engulfment / leukocyte cell-cell adhesion / GP1b-IX-V activation signalling / negative regulation of dopamine metabolic process / SMAD binding / receptor clustering / cortical cytoskeleton / endodermal cell differentiation / amyloid-beta clearance / semaphorin-plexin signaling pathway / RHO GTPases activate PAKs / tertiary granule membrane / cellular response to low-density lipoprotein particle stimulus / ficolin-1-rich granule membrane / plasma membrane raft / cilium assembly / mitotic spindle assembly / positive regulation of protein targeting to membrane / Integrin cell surface interactions / potassium channel regulator activity / endothelial cell migration / : / specific granule membrane / positive regulation of superoxide anion generation / cell adhesion molecule binding / heat shock protein binding / neutrophil chemotaxis / release of sequestered calcium ion into cytosol / positive regulation of substrate adhesion-dependent cell spreading / receptor-mediated endocytosis / protein sequestering activity / regulation of cell migration / cell-matrix adhesion / dendritic shaft / Cell surface interactions at the vascular wall / integrin-mediated signaling pathway / protein localization to plasma membrane / actin filament / establishment of protein localization / mRNA transcription by RNA polymerase II / microglial cell activation / cell-cell adhesion / G protein-coupled receptor binding / negative regulation of protein catabolic process / cerebral cortex development / positive regulation of protein import into nucleus / receptor internalization / small GTPase binding / platelet aggregation / integrin binding / kinase binding / Z disc / positive regulation of angiogenesis / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / positive regulation of nitric oxide biosynthetic process / cell-cell junction / actin filament binding / Platelet degranulation Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | SOLUTION NMR / torsion angle dynamics | ||||||
 Authors Authors | Chatterjee, D. / Lu, L.Z. / Bhattacharjya, S. | ||||||
| Funding support |  Singapore, 1items Singapore, 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structure of FLN IG21 domain in complex with C-terminal peptide of beta-2 Authors: Chatterjee, D. / Lu, L.Z. / Bhattachrjya, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5xr1.cif.gz 5xr1.cif.gz | 603.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5xr1.ent.gz pdb5xr1.ent.gz | 499.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5xr1.json.gz 5xr1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xr/5xr1 https://data.pdbj.org/pub/pdb/validation_reports/xr/5xr1 ftp://data.pdbj.org/pub/pdb/validation_reports/xr/5xr1 ftp://data.pdbj.org/pub/pdb/validation_reports/xr/5xr1 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 13757.035 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Fusion protein of C-terminal peptide of Integrin beta-2 (residues 752-763, P05107 (ITB2_HUMAN)), and Filamin-A IG21 domain (residues 2228-2321, P21333 (FLNA_HUMAN)). Source: (gene. exp.)  Homo sapiens (human) / Gene: ITGB2, CD18, MFI7, FLNA, FLN, FLN1 Homo sapiens (human) / Gene: ITGB2, CD18, MFI7, FLNA, FLN, FLN1Production host: BAC cloning vector attB-P[acman]-ApR-F-2-5-attB (others) References: UniProt: P05107, UniProt: P21333 |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Type: solution Contents: 300 uM [U-13C; U-15N] FLN IG21 domain, 90% H2O/10% D2O Label: 15N, 13C_ / Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample | Conc.: 300 uM / Component: FLN IG21 domain / Isotopic labeling: [U-13C; U-15N] |
| Sample conditions | Ionic strength: 150 mM / Label: Uniformaly 13C, 15N labeled / pH: 6.2 / Pressure: 1 atm / Temperature: 298 K |
-NMR measurement
| NMR spectrometer | Type: Bruker AVANCE III / Manufacturer: Bruker / Model: AVANCE III / Field strength: 600 MHz |
|---|
- Processing
Processing
| NMR software | Name: CNS / Developer: Brunger A. T. et.al. / Classification: refinement |
|---|---|
| Refinement | Method: torsion angle dynamics / Software ordinal: 1 |
| NMR representative | Selection criteria: lowest energy |
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 200 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller











 PDBj
PDBj














 gel filtration
gel filtration