+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5v8k | ||||||
|---|---|---|---|---|---|---|---|
| Title | Homodimeric reaction center of H. modesticaldum | ||||||
 Components Components |
| ||||||
 Keywords Keywords | PHOTOSYNTHESIS / reaction center / homodimeric | ||||||
| Function / homology |  Function and homology information Function and homology informationthylakoid / photosynthesis / 4 iron, 4 sulfur cluster binding / metal ion binding / membrane Similarity search - Function | ||||||
| Biological species |  Heliobacterium modesticaldum (bacteria) Heliobacterium modesticaldum (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  molecular replacement / Resolution: 2.2 Å molecular replacement / Resolution: 2.2 Å | ||||||
 Authors Authors | Gisriel, C. / Sarrou, I. / Ferlez, B. / Golbeck, J. / Redding, K.E. / Fromme, R. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Science / Year: 2017 Journal: Science / Year: 2017Title: Structure of a symmetric photosynthetic reaction center-photosystem. Authors: Gisriel, C. / Sarrou, I. / Ferlez, B. / Golbeck, J.H. / Redding, K.E. / Fromme, R. #1:  Journal: Nature / Year: 2001 Journal: Nature / Year: 2001Title: Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Authors: Jordan, P. / Fromme, P. / Witt, H.T. / Klukas, O. / Saenger, W. / Krauss, N. #2: Journal: J. Mol. Biol. / Year: 1984 Title: X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. Authors: Deisenhofer, J. / Epp, O. / Miki, K. / Huber, R. / Michel, H. #3:  Journal: Science / Year: 2003 Journal: Science / Year: 2003Title: Crystal structure of the RC-LH1 core complex from Rhodopseudomonas palustris. Authors: Roszak, A.W. / Howard, T.D. / Southall, J. / Gardiner, A.T. / Law, C.J. / Isaacs, N.W. / Cogdell, R.J. #4: Journal: Photosyn. Res. / Year: 2012 Title: Purification of the photosynthetic reaction center from Heliobacterium modesticaldum. Authors: Sarrou, I. / Khan, Z. / Cowgill, J. / Lin, S. / Brune, D. / Romberger, S. / Golbeck, J.H. / Redding, K.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5v8k.cif.gz 5v8k.cif.gz | 502.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5v8k.ent.gz pdb5v8k.ent.gz | 433.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5v8k.json.gz 5v8k.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5v8k_validation.pdf.gz 5v8k_validation.pdf.gz | 9.8 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5v8k_full_validation.pdf.gz 5v8k_full_validation.pdf.gz | 10.1 MB | Display | |
| Data in XML |  5v8k_validation.xml.gz 5v8k_validation.xml.gz | 53.2 KB | Display | |
| Data in CIF |  5v8k_validation.cif.gz 5v8k_validation.cif.gz | 67.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/v8/5v8k https://data.pdbj.org/pub/pdb/validation_reports/v8/5v8k ftp://data.pdbj.org/pub/pdb/validation_reports/v8/5v8k ftp://data.pdbj.org/pub/pdb/validation_reports/v8/5v8k | HTTPS FTP |
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Protein/peptide / Sugars , 3 types, 4 molecules AB

| #1: Protein | Mass: 66920.359 Da / Num. of mol.: 1 / Fragment: reaction center protein / Source method: isolated from a natural source / Source: (natural)  Heliobacterium modesticaldum (bacteria) / References: UniProt: Q1MX24 Heliobacterium modesticaldum (bacteria) / References: UniProt: Q1MX24 |
|---|---|
| #2: Protein/peptide | Mass: 3053.593 Da / Num. of mol.: 1 / Fragment: UNP residues 13-37 / Source method: isolated from a natural source Source: (natural)  Heliobacterium modesticaldum (strain ATCC 51547 / Ice1) (bacteria) Heliobacterium modesticaldum (strain ATCC 51547 / Ice1) (bacteria)Strain: ATCC 51547 / Ice1 / References: UniProt: B0TAT4 |
| #9: Sugar |
-Non-polymers , 8 types, 275 molecules 


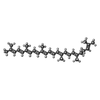








| #3: Chemical | | #4: Chemical | ChemComp-GBF / #5: Chemical | ChemComp-AOH / | #6: Chemical | ChemComp-SF4 / | #7: Chemical | #8: Chemical | #10: Chemical | ChemComp-C4D / | #11: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.47 Å3/Da / Density % sol: 60.7 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: evaporation Details: 0.3 mM HbRC, 100 mM Tris-HCl (ph 8.2), 600 mM NH4Cl, 0.02 % b-DDM, up to 1% n-heptyl-b-D-glucopyranoside, up to 20 % PEG 500 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-BM / Wavelength: 1.0088 Å / Beamline: 19-BM / Wavelength: 1.0088 Å |
| Detector | Type: ADSC QUANTUM 210r / Detector: CCD / Date: Nov 14, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.0088 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→29.1 Å / Num. obs: 61415 / % possible obs: 98 % / Redundancy: 1.9 % / Biso Wilson estimate: 41.31 Å2 / Net I/σ(I): 16.6 |
| Reflection shell | Resolution: 2.2→2.28 Å / Redundancy: 1.7 % / Rmerge(I) obs: 0.48 / % possible all: 89 |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.2→29.08 Å / SU ML: 0.21 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 20.67 / Details: MOLECULAR REPLACEMENT FROM 1JB0 DERIVED MODELS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 54.04 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→29.08 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj










