登録情報 データベース : PDB / ID : 5qu2タイトル Crystal Structure of human Nck SH3.1 in complex with peptide PPPVPNPDY ACE-PRO-PRO-PRO-VAL-PRO-ASN-PRO-ASP-TYR-NH2 Cytoplasmic protein NCK1 キーワード / / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Homo sapiens (ヒト)synthetic construct (人工物) 手法 / / / 解像度 : 1.04 Å データ登録者 Rudolph, M.G. ジャーナル : J.Biol.Chem. / 年 : 2020タイトル : Small molecule AX-024 reduces T cell proliferation independently of CD3ε/Nck1 interaction, which is governed by a domain swap in the Nck1-SH3.1 domain.著者 : Richter, K. / Rufer, A.C. / Muller, M. / Burger, D. / Casagrande, F. / Grossenbacher, T. / Huber, S. / Hug, M.N. / Koldewey, P. / D'Osualdo, A. / Schlatter, D. / Stoll, T. / Rudolph, M.G. 履歴 登録 2019年12月13日 登録サイト / 処理サイト 改定 1.0 2020年2月12日 Provider / タイプ 改定 2.0 2020年2月26日 Group Advisory / Atomic model ... Advisory / Atomic model / Data collection / Database references / Derived calculations / Polymer sequence / Source and taxonomy / Structure summary カテゴリ atom_site / atom_site_anisotrop ... atom_site / atom_site_anisotrop / entity / entity_poly / entity_poly_seq / pdbx_entity_nonpoly / pdbx_entity_src_syn / pdbx_nonpoly_scheme / pdbx_poly_seq_scheme / pdbx_struct_assembly_gen / pdbx_struct_special_symmetry / pdbx_validate_close_contact / pdbx_validate_polymer_linkage / struct_asym / struct_conn / struct_ref_seq / struct_site / struct_site_gen Item _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.group_PDB / _atom_site.label_alt_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.label_seq_id / _atom_site.occupancy / _atom_site.type_symbol / _atom_site_anisotrop.U[1][1] / _atom_site_anisotrop.U[1][2] / _atom_site_anisotrop.U[1][3] / _atom_site_anisotrop.U[2][2] / _atom_site_anisotrop.U[2][3] / _atom_site_anisotrop.U[3][3] / _atom_site_anisotrop.pdbx_auth_asym_id / _atom_site_anisotrop.pdbx_auth_atom_id / _atom_site_anisotrop.pdbx_auth_comp_id / _atom_site_anisotrop.pdbx_auth_seq_id / _atom_site_anisotrop.pdbx_label_alt_id / _atom_site_anisotrop.pdbx_label_asym_id / _atom_site_anisotrop.pdbx_label_atom_id / _atom_site_anisotrop.pdbx_label_comp_id / _atom_site_anisotrop.pdbx_label_seq_id / _atom_site_anisotrop.type_symbol / _entity_poly.pdbx_seq_one_letter_code / _entity_poly.pdbx_seq_one_letter_code_can / _pdbx_entity_src_syn.pdbx_beg_seq_num / _pdbx_entity_src_syn.pdbx_end_seq_num / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_special_symmetry.label_asym_id / _struct_ref_seq.db_align_end / _struct_ref_seq.pdbx_auth_seq_align_end / _struct_ref_seq.seq_align_end 改定 2.1 2021年5月12日 Group / Structure summary / カテゴリ / struct_connItem _pdbx_deposit_group.group_description / _pdbx_deposit_group.group_type ... _pdbx_deposit_group.group_description / _pdbx_deposit_group.group_type / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_ptnr1_label_alt_id / _struct_conn.pdbx_ptnr2_label_alt_id / _struct_conn.pdbx_value_order / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id 改定 2.2 2021年6月30日 Group / カテゴリ / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_ASTM / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year 改定 2.3 2024年4月3日 Group / Database references / Refinement descriptionカテゴリ chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / citation / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_DOI / _database_2.pdbx_database_accession改定 2.4 2024年10月23日 Group カテゴリ / pdbx_modification_feature
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 要素
要素 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) X線回折 /
X線回折 /  シンクロトロン /
シンクロトロン /  分子置換 / 解像度: 1.04 Å
分子置換 / 解像度: 1.04 Å  データ登録者
データ登録者 引用
引用 ジャーナル: J.Biol.Chem. / 年: 2020
ジャーナル: J.Biol.Chem. / 年: 2020 構造の表示
構造の表示 Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク ダウンロード
ダウンロード 5qu2.cif.gz
5qu2.cif.gz PDBx/mmCIF形式
PDBx/mmCIF形式 pdb5qu2.ent.gz
pdb5qu2.ent.gz PDB形式
PDB形式 5qu2.json.gz
5qu2.json.gz PDBx/mmJSON形式
PDBx/mmJSON形式 その他のダウンロード
その他のダウンロード 5qu2_validation.pdf.gz
5qu2_validation.pdf.gz wwPDB検証レポート
wwPDB検証レポート 5qu2_full_validation.pdf.gz
5qu2_full_validation.pdf.gz 5qu2_validation.xml.gz
5qu2_validation.xml.gz 5qu2_validation.cif.gz
5qu2_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/qu/5qu2
https://data.pdbj.org/pub/pdb/validation_reports/qu/5qu2 ftp://data.pdbj.org/pub/pdb/validation_reports/qu/5qu2
ftp://data.pdbj.org/pub/pdb/validation_reports/qu/5qu2 リンク
リンク 集合体
集合体
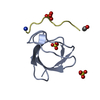

 要素
要素 Homo sapiens (ヒト) / 遺伝子: NCK1, NCK / プラスミド: PET28a(+) / 発現宿主:
Homo sapiens (ヒト) / 遺伝子: NCK1, NCK / プラスミド: PET28a(+) / 発現宿主: 
 X線回折 / 使用した結晶の数: 1
X線回折 / 使用した結晶の数: 1  試料調製
試料調製 シンクロトロン / サイト:
シンクロトロン / サイト:  SLS
SLS  / ビームライン: X10SA / 波長: 1.00005 Å
/ ビームライン: X10SA / 波長: 1.00005 Å 解析
解析 分子置換
分子置換 ムービー
ムービー コントローラー
コントローラー



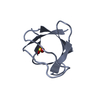


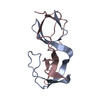







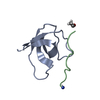

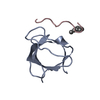



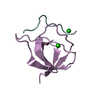
 PDBj
PDBj





















