[English] 日本語
 Yorodumi
Yorodumi- PDB-5ndd: Crystal structure of a thermostabilised human protease-activated ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ndd | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of a thermostabilised human protease-activated receptor-2 (PAR2) in complex with AZ8838 at 2.8 angstrom resolution | ||||||
 Components Components | Lysozyme,Proteinase-activated receptor 2,Soluble cytochrome b562,Proteinase-activated receptor 2 | ||||||
 Keywords Keywords | MEMBRANE PROTEIN / GPCR / 7TM | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of neutrophil mediated killing of gram-negative bacterium / positive regulation of renin secretion into blood stream / positive regulation of eosinophil degranulation / leukocyte proliferation / mature conventional dendritic cell differentiation / positive regulation of glomerular filtration / regulation of chemokine (C-X-C motif) ligand 2 production / negative regulation of toll-like receptor 3 signaling pathway / positive regulation of toll-like receptor 3 signaling pathway / thrombin-activated receptor activity ...positive regulation of neutrophil mediated killing of gram-negative bacterium / positive regulation of renin secretion into blood stream / positive regulation of eosinophil degranulation / leukocyte proliferation / mature conventional dendritic cell differentiation / positive regulation of glomerular filtration / regulation of chemokine (C-X-C motif) ligand 2 production / negative regulation of toll-like receptor 3 signaling pathway / positive regulation of toll-like receptor 3 signaling pathway / thrombin-activated receptor activity / positive regulation of toll-like receptor 2 signaling pathway / positive regulation of actin filament depolymerization / cell-cell junction maintenance / positive regulation of toll-like receptor 4 signaling pathway / T cell activation involved in immune response / positive regulation of pseudopodium assembly / positive regulation of leukocyte chemotaxis / positive regulation of cytokine production involved in immune response / negative regulation of chemokine production / neutrophil activation / positive regulation of phagocytosis, engulfment / positive regulation of chemotaxis / establishment of endothelial barrier / positive regulation of positive chemotaxis / regulation of canonical NF-kappaB signal transduction / regulation of JNK cascade / negative regulation of JNK cascade / leukocyte migration / regulation of blood coagulation / positive regulation of Rho protein signal transduction / pseudopodium / positive regulation of interleukin-10 production / G-protein alpha-subunit binding / positive regulation of GTPase activity / negative regulation of tumor necrosis factor-mediated signaling pathway / viral release from host cell by cytolysis / positive regulation of chemokine production / positive regulation of superoxide anion generation / peptidoglycan catabolic process / Peptide ligand-binding receptors / positive regulation of interleukin-1 beta production / positive regulation of interleukin-8 production / positive regulation of JNK cascade / electron transport chain / negative regulation of insulin secretion / G protein-coupled receptor activity / positive regulation of interleukin-6 production / vasodilation / positive regulation of type II interferon production / cell wall macromolecule catabolic process / blood coagulation / lysozyme / lysozyme activity / G-protein beta-subunit binding / signaling receptor activity / positive regulation of cytosolic calcium ion concentration / protease binding / defense response to virus / G alpha (q) signalling events / host cell cytoplasm / early endosome / electron transfer activity / periplasmic space / positive regulation of ERK1 and ERK2 cascade / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of canonical NF-kappaB signal transduction / defense response to bacterium / positive regulation of cell migration / iron ion binding / G protein-coupled receptor signaling pathway / inflammatory response / signaling receptor binding / innate immune response / heme binding / Golgi apparatus / positive regulation of transcription by RNA polymerase II / plasma membrane Similarity search - Function | ||||||
| Biological species |  Enterobacteria phage T4 (virus) Enterobacteria phage T4 (virus) Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.801 Å MOLECULAR REPLACEMENT / Resolution: 2.801 Å | ||||||
 Authors Authors | Cheng, R.K.Y. / Fiez-Vandal, C. / Schlenker, O. / Edman, K. / Aggeler, B. / Brown, D.G. / Brown, G. / Cooke, R.M. / Dumelin, C.E. / Dore, A.S. ...Cheng, R.K.Y. / Fiez-Vandal, C. / Schlenker, O. / Edman, K. / Aggeler, B. / Brown, D.G. / Brown, G. / Cooke, R.M. / Dumelin, C.E. / Dore, A.S. / Geschwindner, S. / Grebner, C. / Hermansson, N.-O. / Jazayeri, A. / Johansson, P. / Leong, L. / Prihandoko, R. / Rappas, M. / Soutter, H. / Snijder, A. / Sundstrom, L. / Tehan, B. / Thornton, P. / Troast, D. / Wiggin, G. / Zhukov, A. / Marshall, F.H. / Dekker, N. | ||||||
 Citation Citation |  Journal: Nature / Year: 2017 Journal: Nature / Year: 2017Title: Structural insight into allosteric modulation of protease-activated receptor 2. Authors: Cheng, R.K.Y. / Fiez-Vandal, C. / Schlenker, O. / Edman, K. / Aggeler, B. / Brown, D.G. / Brown, G.A. / Cooke, R.M. / Dumelin, C.E. / Dore, A.S. / Geschwindner, S. / Grebner, C. / ...Authors: Cheng, R.K.Y. / Fiez-Vandal, C. / Schlenker, O. / Edman, K. / Aggeler, B. / Brown, D.G. / Brown, G.A. / Cooke, R.M. / Dumelin, C.E. / Dore, A.S. / Geschwindner, S. / Grebner, C. / Hermansson, N.O. / Jazayeri, A. / Johansson, P. / Leong, L. / Prihandoko, R. / Rappas, M. / Soutter, H. / Snijder, A. / Sundstrom, L. / Tehan, B. / Thornton, P. / Troast, D. / Wiggin, G. / Zhukov, A. / Marshall, F.H. / Dekker, N. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ndd.cif.gz 5ndd.cif.gz | 129.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ndd.ent.gz pdb5ndd.ent.gz | 96.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ndd.json.gz 5ndd.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nd/5ndd https://data.pdbj.org/pub/pdb/validation_reports/nd/5ndd ftp://data.pdbj.org/pub/pdb/validation_reports/nd/5ndd ftp://data.pdbj.org/pub/pdb/validation_reports/nd/5ndd | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5ndzC  5nj6C  3vw7S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 69558.898 Da / Num. of mol.: 1 Mutation: G89A, H108A, G157A, M166L, Y174A, V176E, N222Q, M268A,I289A, L293A Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Enterobacteria phage T4 (virus), (gene. exp.) Enterobacteria phage T4 (virus), (gene. exp.)  Homo sapiens (human), (gene. exp.) Homo sapiens (human), (gene. exp.)  Gene: e, T4Tp126, F2RL1, GPR11, PAR2, cybC / Plasmid: pFASTBAC / Cell line (production host): Sf9 / Production host:  References: UniProt: D9IEF7, UniProt: P55085, UniProt: P0ABE7, UniProt: P00720*PLUS, lysozyme |
|---|---|
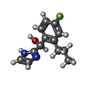
| #2: Chemical | ChemComp-8TZ / (~{ |
| #3: Chemical | ChemComp-NA / |
| #4: Chemical | ChemComp-PO4 / |
| #5: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.04 Å3/Da / Density % sol: 59.52 % / Description: Needle-shaped crystals (on average 0.1mm long) |
|---|---|
| Crystal grow | Temperature: 293 K / Method: lipidic cubic phase / pH: 5.8 Details: 0.1 M sodium citrate/citrate acid pH 5.5-6.2, 0.2 M ammonium phosphate dibasic, 38-43 % (w/v) PEG400 and 1 mM AZ8838 PH range: 5.5-6.2 / Temp details: Constant |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I24 / Wavelength: 0.96859 Å / Beamline: I24 / Wavelength: 0.96859 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Jun 5, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.96859 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→34.34 Å / Num. obs: 17995 / % possible obs: 97.5 % / Redundancy: 3.4 % / Biso Wilson estimate: 24.2 Å2 / CC1/2: 0.989 / Rmerge(I) obs: 0.134 / Rpim(I) all: 0.11 / Net I/σ(I): 6.5 |
| Reflection shell | Resolution: 2.8→2.95 Å / Redundancy: 3.4 % / Rmerge(I) obs: 0.633 / Mean I/σ(I) obs: 1.7 / Num. unique obs: 2671 / CC1/2: 0.692 / Rpim(I) all: 0.522 / % possible all: 98.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3VW7 Resolution: 2.801→34.336 Å / SU ML: 0.33 / Cross valid method: FREE R-VALUE / σ(F): 1.92 / Phase error: 30.31
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.801→34.336 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj