| Entry | Database: PDB / ID: 5m9z
|
|---|
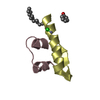
| Title | Second zinc-binding domain from yeast Pcf11 |
|---|
 Components Components | Protein PCF11 |
|---|
 Keywords Keywords | RNA BINDING PROTEIN / zinc-binding / mRNA / RNA processing |
|---|
| Function / homology |  Function and homology information Function and homology information
termination of RNA polymerase II transcription, poly(A)-coupled / termination of RNA polymerase II transcription, exosome-dependent / mRNA cleavage factor complex / mRNA 3'-end processing / termination of RNA polymerase II transcription / RNA polymerase II complex binding / mRNA binding / nucleus / cytosol / cytoplasmSimilarity search - Function : / Pfc11 Rna14/15 interacting domain / Subunit of cleavage factor IA Pcf11, C-terminal / Protein PCF11-like / : / : / Pcf11, Clp1-interaction domain / Pcf11, C-terminal domain / CID domain / RPR ...: / Pfc11 Rna14/15 interacting domain / Subunit of cleavage factor IA Pcf11, C-terminal / Protein PCF11-like / : / : / Pcf11, Clp1-interaction domain / Pcf11, C-terminal domain / CID domain / RPR / CID domain / CID domain profile. / ENTH/VHSSimilarity search - Domain/homology |
|---|
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
|---|
| Method | SOLUTION NMR / simulated annealing |
|---|
 Authors Authors | Mackereth, C. |
|---|
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2017 Journal: Nucleic Acids Res. / Year: 2017
Title: Distinct roles of Pcf11 zinc-binding domains in pre-mRNA 3'-end processing.
Authors: Guegueniat, J. / Dupin, A.F. / Stojko, J. / Beaurepaire, L. / Cianferani, S. / Mackereth, C.D. / Minvielle-Sebastia, L. / Fribourg, S. |
|---|
| History | | Deposition | Nov 2, 2016 | Deposition site: PDBE / Processing site: PDBE |
|---|
| Revision 1.0 | Aug 2, 2017 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Oct 11, 2017 | Group: Database references / Category: citation / citation_author
Item: _citation.journal_volume / _citation.page_first ..._citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title |
|---|
| Revision 1.2 | Jan 31, 2018 | Group: Data collection / Category: pdbx_nmr_spectrometer / Item: _pdbx_nmr_spectrometer.model |
|---|
| Revision 1.3 | May 8, 2019 | Group: Data collection / Category: pdbx_nmr_software / Item: _pdbx_nmr_software.name |
|---|
| Revision 1.4 | May 15, 2024 | Group: Data collection / Database references / Derived calculations
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_nmr_spectrometer / struct_conn
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_nmr_spectrometer.model / _struct_conn.pdbx_dist_value |
|---|
|
|---|
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 Authors
Authors Citation
Citation Journal: Nucleic Acids Res. / Year: 2017
Journal: Nucleic Acids Res. / Year: 2017 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5m9z.cif.gz
5m9z.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5m9z.ent.gz
pdb5m9z.ent.gz PDB format
PDB format 5m9z.json.gz
5m9z.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 5m9z_validation.pdf.gz
5m9z_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 5m9z_full_validation.pdf.gz
5m9z_full_validation.pdf.gz 5m9z_validation.xml.gz
5m9z_validation.xml.gz 5m9z_validation.cif.gz
5m9z_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/m9/5m9z
https://data.pdbj.org/pub/pdb/validation_reports/m9/5m9z ftp://data.pdbj.org/pub/pdb/validation_reports/m9/5m9z
ftp://data.pdbj.org/pub/pdb/validation_reports/m9/5m9z Links
Links Assembly
Assembly
 Components
Components

 Sample preparation
Sample preparation Movie
Movie Controller
Controller












 PDBj
PDBj


 HSQC
HSQC