[English] 日本語
 Yorodumi
Yorodumi- PDB-5laq: Crystal structure of human phosphodiesterase 4B catalytic domain ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5laq | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human phosphodiesterase 4B catalytic domain with inhibitor NPD-001 | ||||||
 Components Components | cAMP-specific 3',5'-cyclic phosphodiesterase 4B,cAMP-specific 3',5'-cyclic phosphodiesterase 4B | ||||||
 Keywords Keywords | HYDROLASE / Phosphodiesterase / cAMP / alternative splicing | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of adenylate cyclase-activating adrenergic receptor signaling pathway / gamma-tubulin complex / negative regulation of relaxation of cardiac muscle / 3',5'-cyclic-AMP phosphodiesterase / neutrophil homeostasis / gamma-tubulin binding / regulation of cardiac muscle cell contraction / regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / voltage-gated calcium channel complex / leukocyte migration ...negative regulation of adenylate cyclase-activating adrenergic receptor signaling pathway / gamma-tubulin complex / negative regulation of relaxation of cardiac muscle / 3',5'-cyclic-AMP phosphodiesterase / neutrophil homeostasis / gamma-tubulin binding / regulation of cardiac muscle cell contraction / regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / voltage-gated calcium channel complex / leukocyte migration / cAMP catabolic process / 3',5'-cyclic-GMP phosphodiesterase activity / excitatory synapse / DARPP-32 events / 3',5'-cyclic-AMP phosphodiesterase activity / cAMP-mediated signaling / cAMP binding / cellular response to epinephrine stimulus / neutrophil chemotaxis / positive regulation of interleukin-2 production / calcium channel regulator activity / Z disc / cellular response to xenobiotic stimulus / positive regulation of type II interferon production / synaptic vesicle / T cell receptor signaling pathway / cellular response to lipopolysaccharide / dendritic spine / transmembrane transporter binding / postsynaptic density / centrosome / metal ion binding / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | ||||||
 Authors Authors | Singh, A.K. / Brown, D.G. | ||||||
 Citation Citation |  Journal: J. Med. Chem. / Year: 2018 Journal: J. Med. Chem. / Year: 2018Title: Targeting a Subpocket in Trypanosoma brucei Phosphodiesterase B1 (TbrPDEB1) Enables the Structure-Based Discovery of Selective Inhibitors with Trypanocidal Activity. Authors: Blaazer, A.R. / Singh, A.K. / de Heuvel, E. / Edink, E. / Orrling, K.M. / Veerman, J.J.N. / van den Bergh, T. / Jansen, C. / Balasubramaniam, E. / Mooij, W.J. / Custers, H. / Sijm, M. / ...Authors: Blaazer, A.R. / Singh, A.K. / de Heuvel, E. / Edink, E. / Orrling, K.M. / Veerman, J.J.N. / van den Bergh, T. / Jansen, C. / Balasubramaniam, E. / Mooij, W.J. / Custers, H. / Sijm, M. / Tagoe, D.N.A. / Kalejaiye, T.D. / Munday, J.C. / Tenor, H. / Matheeussen, A. / Wijtmans, M. / Siderius, M. / de Graaf, C. / Maes, L. / de Koning, H.P. / Bailey, D.S. / Sterk, G.J. / de Esch, I.J.P. / Brown, D.G. / Leurs, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5laq.cif.gz 5laq.cif.gz | 90.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5laq.ent.gz pdb5laq.ent.gz | 65 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5laq.json.gz 5laq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5laq_validation.pdf.gz 5laq_validation.pdf.gz | 699.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5laq_full_validation.pdf.gz 5laq_full_validation.pdf.gz | 701 KB | Display | |
| Data in XML |  5laq_validation.xml.gz 5laq_validation.xml.gz | 14.8 KB | Display | |
| Data in CIF |  5laq_validation.cif.gz 5laq_validation.cif.gz | 20.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/la/5laq https://data.pdbj.org/pub/pdb/validation_reports/la/5laq ftp://data.pdbj.org/pub/pdb/validation_reports/la/5laq ftp://data.pdbj.org/pub/pdb/validation_reports/la/5laq | HTTPS FTP |
-Related structure data
| Related structure data |  5g2bC  5g57C  5g5vC  5l8cC  5l8yC  5l9hC  5lboC  3g45S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 48442.699 Da / Num. of mol.: 1 Fragment: UNP residues 241-659, Catalytic domain UNP residues 305-659 Source method: isolated from a genetically manipulated source Details: linker is from PDE4C that was swapped to replace the natural linker Source: (gene. exp.)  Homo sapiens (human) / Gene: PDE4B, DPDE4 / Cell line (production host): Sf21 / Production host: Homo sapiens (human) / Gene: PDE4B, DPDE4 / Cell line (production host): Sf21 / Production host:  References: UniProt: Q07343, 3',5'-cyclic-AMP phosphodiesterase |
|---|
-Non-polymers , 5 types, 91 molecules 


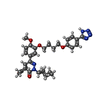





| #2: Chemical | ChemComp-ZN / | ||||
|---|---|---|---|---|---|
| #3: Chemical | ChemComp-MG / | ||||
| #4: Chemical | | #5: Chemical | ChemComp-6M5 / ( | #6: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.35 Å3/Da / Density % sol: 47.6 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 4.6 Details: 50 mM calcium acetate, 20% PEG 400, 100 mM sodium acetate |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.97623 Å / Beamline: I03 / Wavelength: 0.97623 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Apr 28, 2016 / Details: CRL |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97623 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→82.91 Å / Num. obs: 17941 / % possible obs: 100 % / Redundancy: 8.5 % / CC1/2: 0.998 / Rmerge(I) obs: 0.129 / Net I/σ(I): 13 |
| Reflection shell | Resolution: 2.4→2.53 Å / Redundancy: 6.1 % / Rmerge(I) obs: 0.78 / Mean I/σ(I) obs: 2.3 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3G45 Resolution: 2.4→82.91 Å / Cor.coef. Fo:Fc: 0.962 / Cor.coef. Fo:Fc free: 0.931 / SU B: 8.475 / SU ML: 0.193 / Cross valid method: THROUGHOUT / ESU R: 0.295 / ESU R Free: 0.24 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U values refined individually
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 51.062 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.4→82.91 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj






