| Entry | Database: PDB / ID: 5l23
|
|---|
| Title | Crystal structure of the complex between the N-terminal SH3 domain of CrkII and a proline-rich ligand |
|---|
 Components Components | - Adapter molecule crk
- C3G derived peptide
|
|---|
 Keywords Keywords | PROTEIN BINDING / SH3 / acetylation / amidation / Crkii / C3G |
|---|
| Function / homology |  Function and homology information Function and homology information
PTK6 Regulates RHO GTPases, RAS GTPase and MAP kinases / ARMS-mediated activation / MET activates RAP1 and RAC1 / MET receptor recycling / response to hepatocyte growth factor / cellular response to endothelin / helper T cell diapedesis / cerebellar neuron development / response to cholecystokinin / Downstream signal transduction ...PTK6 Regulates RHO GTPases, RAS GTPase and MAP kinases / ARMS-mediated activation / MET activates RAP1 and RAC1 / MET receptor recycling / response to hepatocyte growth factor / cellular response to endothelin / helper T cell diapedesis / cerebellar neuron development / response to cholecystokinin / Downstream signal transduction / regulation of leukocyte migration / Rap protein signal transduction / postsynaptic specialization assembly / protein phosphorylated amino acid binding / regulation of T cell migration / regulation of dendrite development / p130Cas linkage to MAPK signaling for integrins / response to peptide / regulation of cell junction assembly / positive regulation of skeletal muscle acetylcholine-gated channel clustering / response to yeast / Regulation of signaling by CBL / nerve growth factor signaling pathway / negative regulation of wound healing / Regulation of actin dynamics for phagocytic cup formation / reelin-mediated signaling pathway / negative regulation of cell motility / VEGFA-VEGFR2 Pathway / establishment of endothelial barrier / negative regulation of natural killer cell mediated cytotoxicity / protein localization to membrane / MET activates RAP1 and RAC1 / regulation of GTPase activity / cellular response to insulin-like growth factor stimulus / Frs2-mediated activation / positive regulation of smooth muscle cell migration / enzyme-linked receptor protein signaling pathway / establishment of cell polarity / regulation of cell adhesion mediated by integrin / dendrite development / positive regulation of Rac protein signal transduction / ephrin receptor signaling pathway / RAC3 GTPase cycle / Erythropoietin activates RAS / cytoskeletal protein binding / cellular response to transforming growth factor beta stimulus / insulin-like growth factor receptor binding / cellular response to cAMP / signaling adaptor activity / ephrin receptor binding / positive regulation of substrate adhesion-dependent cell spreading / phosphotyrosine residue binding / positive regulation of GTPase activity / Downstream signal transduction / cellular response to nitric oxide / SH2 domain binding / cell surface receptor protein tyrosine kinase signaling pathway / protein tyrosine kinase binding / guanyl-nucleotide exchange factor activity / hippocampus development / cell chemotaxis / regulation of actin cytoskeleton organization / Regulation of signaling by CBL / positive regulation of JNK cascade / cellular response to nerve growth factor stimulus / neuromuscular junction / cerebral cortex development / positive regulation of neuron projection development / response to hydrogen peroxide / SH3 domain binding / lipid metabolic process / neuron migration / nervous system development / actin cytoskeleton / signaling receptor complex adaptor activity / regulation of cell shape / actin cytoskeleton organization / scaffold protein binding / Ras protein signal transduction / early endosome / cell population proliferation / intracellular membrane-bounded organelle / ubiquitin protein ligase binding / signal transduction / protein-containing complex / membrane / plasma membrane / cytosol / cytoplasmSimilarity search - Function CRK, N-terminal SH3 domain / CRK, C-terminal SH3 domain / : / Ras guanine-nucleotide exchange factor, conserved site / Ras Guanine-nucleotide exchange factors domain signature. / RasGEF N-terminal motif / Guanine nucleotide exchange factor for Ras-like GTPases; N-terminal motif / Ras-like guanine nucleotide exchange factor, N-terminal / Ras-like guanine nucleotide exchange factor / Ras guanine-nucleotide exchange factors N-terminal domain profile. ...CRK, N-terminal SH3 domain / CRK, C-terminal SH3 domain / : / Ras guanine-nucleotide exchange factor, conserved site / Ras Guanine-nucleotide exchange factors domain signature. / RasGEF N-terminal motif / Guanine nucleotide exchange factor for Ras-like GTPases; N-terminal motif / Ras-like guanine nucleotide exchange factor, N-terminal / Ras-like guanine nucleotide exchange factor / Ras guanine-nucleotide exchange factors N-terminal domain profile. / Ras guanine nucleotide exchange factor domain superfamily / Ras guanine-nucleotide exchange factor, catalytic domain superfamily / RasGEF domain / Ras guanine-nucleotide exchange factors catalytic domain profile. / Guanine nucleotide exchange factor for Ras-like small GTPases / Ras guanine-nucleotide exchange factors catalytic domain / Variant SH3 domain / SH3 Domains / SH3 domain / SH2 domain / Src homology 2 (SH2) domain profile. / Src homology 2 domains / SH2 domain / SH2 domain superfamily / Src homology 3 domains / SH3 type barrels. / SH3-like domain superfamily / Src homology 3 (SH3) domain profile. / SH3 domain / Roll / Mainly BetaSimilarity search - Domain/homology |
|---|
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.77 Å MOLECULAR REPLACEMENT / Resolution: 1.77 Å |
|---|
 Authors Authors | Bhatt, V.S. / Krieger, I. / Sacchettini, J. / Cho, J.-H. |
|---|
 Citation Citation |  Journal: To Be Published Journal: To Be Published
Title: Crystal structure of the complex between the N-terminal SH3 domain of CrkII and a proline-rich ligand
Authors: Bhatt, V.S. / Krieger, I. / Sacchettini, J. / Cho, J.-H. |
|---|
| History | | Deposition | Jul 30, 2016 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Sep 7, 2016 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Nov 15, 2017 | Group: Derived calculations / Source and taxonomy / Category: entity_src_gen / pdbx_struct_oper_list
Item: _entity_src_gen.pdbx_host_org_ncbi_taxonomy_id / _entity_src_gen.pdbx_host_org_scientific_name / _pdbx_struct_oper_list.symmetry_operation |
|---|
| Revision 1.2 | Oct 4, 2023 | Group: Data collection / Database references / Refinement description
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession |
|---|
| Revision 1.3 | Oct 23, 2024 | Group: Structure summary / Category: pdbx_entry_details / pdbx_modification_feature |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.77 Å
MOLECULAR REPLACEMENT / Resolution: 1.77 Å  Authors
Authors Citation
Citation Journal: To Be Published
Journal: To Be Published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5l23.cif.gz
5l23.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5l23.ent.gz
pdb5l23.ent.gz PDB format
PDB format 5l23.json.gz
5l23.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/l2/5l23
https://data.pdbj.org/pub/pdb/validation_reports/l2/5l23 ftp://data.pdbj.org/pub/pdb/validation_reports/l2/5l23
ftp://data.pdbj.org/pub/pdb/validation_reports/l2/5l23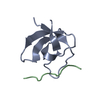
 Links
Links Assembly
Assembly
 Components
Components


 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation ROTATING ANODE / Type: RIGAKU / Wavelength: 1.54 Å
ROTATING ANODE / Type: RIGAKU / Wavelength: 1.54 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller









 PDBj
PDBj
















