[English] 日本語
 Yorodumi
Yorodumi- PDB-5hwi: Crystal structure of selenomethionine labelled gama glutamyl cycl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5hwi | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of selenomethionine labelled gama glutamyl cyclotransferease specific to glutathione from yeast | ||||||
 Components Components | Glutathione-specific gamma-glutamylcyclotransferase | ||||||
 Keywords Keywords | TRANSFERASE / ChaC / GCG1 / Yer163c / glutathione / oxo-proline | ||||||
| Function / homology |  Function and homology information Function and homology informationglutathione-specific gamma-glutamylcyclotransferase / Glutathione synthesis and recycling / glutathione specific gamma-glutamylcyclotransferase activity / gamma-glutamylcyclotransferase activity / glutathione catabolic process / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 1.755 Å SAD / Resolution: 1.755 Å | ||||||
 Authors Authors | Kaur, A. / Gautam, R. / Srivastava, R. / Chandel, A. / Kumar, A. / Karthikeyan, S. / Bachhawat, A.K. | ||||||
| Funding support |  India, 1items India, 1items
| ||||||
 Citation Citation |  Journal: J. Biol. Chem. / Year: 2017 Journal: J. Biol. Chem. / Year: 2017Title: ChaC2, an Enzyme for Slow Turnover of Cytosolic Glutathione Authors: Kaur, A. / Gautam, R. / Srivastava, R. / Chandel, A. / Kumar, A. / Karthikeyan, S. / Bachhawat, A.K. | ||||||
| History |
|
- Structure visualization
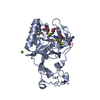
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5hwi.cif.gz 5hwi.cif.gz | 114.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5hwi.ent.gz pdb5hwi.ent.gz | 88.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5hwi.json.gz 5hwi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hw/5hwi https://data.pdbj.org/pub/pdb/validation_reports/hw/5hwi ftp://data.pdbj.org/pub/pdb/validation_reports/hw/5hwi ftp://data.pdbj.org/pub/pdb/validation_reports/hw/5hwi | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 27773.613 Da / Num. of mol.: 1 / Mutation: S42M,L77M,A141M,V176M Source method: isolated from a genetically manipulated source Details: Mutated original sequence to Methionine for generating Se-Met protein Source: (gene. exp.)   References: UniProt: P32656, Transferases; Acyltransferases; Aminoacyltransferases |
|---|---|
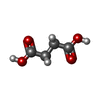
| #2: Chemical | ChemComp-SIN / |
| #3: Chemical | ChemComp-GOL / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 51 % / Description: Rectangular Plate |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 1 M Succinic acid, 0.1 M HEPES, 1%(w/v) PEG MME-2000 PH range: 6.8 - 7.2 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM14 / Wavelength: 0.9787 Å / Beamline: BM14 / Wavelength: 0.9787 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Sep 24, 2014 / Details: MIRROR |
| Radiation | Monochromator: SI III / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9787 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→50 Å / Num. obs: 25966 / % possible obs: 96.3 % / Observed criterion σ(I): -3 / Redundancy: 34.9 % / Biso Wilson estimate: 26.84 Å2 / CC1/2: 0.96 / Rmerge(I) obs: 0.1 / Net I/σ(I): 41.9 |
| Reflection shell | Resolution: 1.75→1.78 Å / Redundancy: 15.2 % / Rmerge(I) obs: 0.93 / Mean I/σ(I) obs: 2.1 / CC1/2: 0.83 / % possible all: 76.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 1.755→37.4 Å / SU ML: 0.14 / Cross valid method: FREE R-VALUE / σ(F): 0.02 / Phase error: 22.43 / Stereochemistry target values: ML SAD / Resolution: 1.755→37.4 Å / SU ML: 0.14 / Cross valid method: FREE R-VALUE / σ(F): 0.02 / Phase error: 22.43 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.755→37.4 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 23.1313 Å / Origin y: 57.3614 Å / Origin z: 16.9669 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller












 PDBj
PDBj