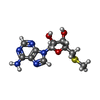
Entry Database : PDB / ID : 5fa5Title Crystal Structure of PRMT5:MEP50 in complex with MTA and H4 peptide Histone H4 Methylosome protein 50 Protein arginine N-methyltransferase 5 Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / / Resolution : 2.34 Å Authors Sprague, E.R. / McNamara, J.T. Journal : Science / Year : 2016Title : Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5.Authors: Mavrakis, K.J. / McDonald, E.R. / Schlabach, M.R. / Billy, E. / Hoffman, G.R. / deWeck, A. / Ruddy, D.A. / Venkatesan, K. / Yu, J. / McAllister, G. / Stump, M. / deBeaumont, R. / Ho, S. / ... Authors : Mavrakis, K.J. / McDonald, E.R. / Schlabach, M.R. / Billy, E. / Hoffman, G.R. / deWeck, A. / Ruddy, D.A. / Venkatesan, K. / Yu, J. / McAllister, G. / Stump, M. / deBeaumont, R. / Ho, S. / Yue, Y. / Liu, Y. / Yan-Neale, Y. / Yang, G. / Lin, F. / Yin, H. / Gao, H. / Kipp, D.R. / Zhao, S. / McNamara, J.T. / Sprague, E.R. / Zheng, B. / Lin, Y. / Cho, Y.S. / Gu, J. / Crawford, K. / Ciccone, D. / Vitari, A.C. / Lai, A. / Capka, V. / Hurov, K. / Porter, J.A. / Tallarico, J. / Mickanin, C. / Lees, E. / Pagliarini, R. / Keen, N. / Schmelzle, T. / Hofmann, F. / Stegmeier, F. / Sellers, W.R. History Deposition Dec 10, 2015 Deposition site / Processing site Revision 1.0 Feb 24, 2016 Provider / Type Revision 1.1 Mar 9, 2016 Group Revision 1.2 Mar 23, 2016 Group Revision 1.3 Sep 27, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / citation / database_2 / pdbx_initial_refinement_model / pdbx_struct_oper_list Item _citation.journal_id_CSD / _database_2.pdbx_DOI ... _citation.journal_id_CSD / _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_oper_list.symmetry_operation
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.34 Å
molecular replacement / Resolution: 2.34 Å  Authors
Authors Citation
Citation Journal: Science / Year: 2016
Journal: Science / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5fa5.cif.gz
5fa5.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5fa5.ent.gz
pdb5fa5.ent.gz PDB format
PDB format 5fa5.json.gz
5fa5.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/fa/5fa5
https://data.pdbj.org/pub/pdb/validation_reports/fa/5fa5 ftp://data.pdbj.org/pub/pdb/validation_reports/fa/5fa5
ftp://data.pdbj.org/pub/pdb/validation_reports/fa/5fa5
 Links
Links Assembly
Assembly



 Components
Components Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host:
Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host: 
 Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host:
Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host: 
 Homo sapiens (human) / References: UniProt: P62805
Homo sapiens (human) / References: UniProt: P62805 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 17-ID / Wavelength: 1 Å
/ Beamline: 17-ID / Wavelength: 1 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller












 PDBj
PDBj