+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5eco | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of FIN219-FIP1 complex with JA, Leu and Mg | ||||||
 Components Components |
| ||||||
 Keywords Keywords | LIGASE/TRANSFERASE / Jasmonate-amido synthetase / Glutathione S-transferase / Ligase-Transferase complex | ||||||
| Function / homology |  Function and homology information Function and homology informationjasmonoyl-L-amino acid ligase / jasmonoyl-L-amino acid ligase activity / regulation of response to red or far red light / cellular response to auxin stimulus / induced systemic resistance, jasmonic acid mediated signaling pathway / toxin catabolic process / L-leucine binding / protein adenylylation / response to mycotoxin / glutathione binding ...jasmonoyl-L-amino acid ligase / jasmonoyl-L-amino acid ligase activity / regulation of response to red or far red light / cellular response to auxin stimulus / induced systemic resistance, jasmonic acid mediated signaling pathway / toxin catabolic process / L-leucine binding / protein adenylylation / response to mycotoxin / glutathione binding / apoplast / response to UV-B / amino acid binding / response to gravity / glutathione transferase / glutathione transferase activity / glutathione metabolic process / chloroplast / enzyme binding / mitochondrion / ATP binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Chen, C.Y. / Cheng, Y.S. | ||||||
 Citation Citation |  Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2017 Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2017Title: Structural basis of jasmonate-amido synthetase FIN219 in complex with glutathione S-transferase FIP1 during the JA signal regulation Authors: Chen, C.Y. / Ho, S.S. / Kuo, T.Y. / Hsieh, H.L. / Cheng, Y.S. #1:  Journal: Science / Year: 2012 Journal: Science / Year: 2012Title: Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Authors: Westfall, C.S. / Zubieta, C. / Herrmann, J. / Kapp, U. / Nanao, M.H. / Jez, J.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5eco.cif.gz 5eco.cif.gz | 461.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5eco.ent.gz pdb5eco.ent.gz | 364.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5eco.json.gz 5eco.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ec/5eco https://data.pdbj.org/pub/pdb/validation_reports/ec/5eco ftp://data.pdbj.org/pub/pdb/validation_reports/ec/5eco ftp://data.pdbj.org/pub/pdb/validation_reports/ec/5eco | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5echC  5eciC  5eckC  5eclC  5ecmC  5ecnC  5ecpC  5ecqC  5ecrC  5ecsC  5gzzC  4eplS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 6 molecules ADBCEF
| #1: Protein | Mass: 64416.199 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: Q9SKE2, Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) #2: Protein | Mass: 25867.312 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 5 types, 2317 molecules 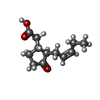


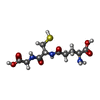





| #3: Chemical | | #4: Chemical | #5: Chemical | ChemComp-MG / | #6: Chemical | ChemComp-GSH / #7: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.26 Å3/Da / Density % sol: 45.54 % |
|---|---|
| Crystal grow | Temperature: 297 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: 0.2 M sodium acetate trihydrate, 0.1 M Tris-HCl, 30%(w/v) PEG 3000 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSRRC NSRRC  / Beamline: BL13C1 / Wavelength: 0.9762-0.97622 / Beamline: BL13C1 / Wavelength: 0.9762-0.97622 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RAYONIX MX-300 / Detector: CCD / Date: Nov 8, 2012 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: LAUE / Monochromatic (M) / Laue (L): L / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.8→50 Å / Num. obs: 174895 / % possible obs: 94.9 % / Redundancy: 1.8 % / Biso Wilson estimate: 8.57 Å2 / Rmerge(I) obs: 0.066 / Χ2: 0.996 / Net I/av σ(I): 8.962 / Net I/σ(I): 6.9 / Num. measured all: 316315 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Redundancy: 1.8 % / Rejects: _
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4EPL Resolution: 1.8→23.483 Å / SU ML: 0.15 / Cross valid method: FREE R-VALUE / σ(F): 1.57 / Phase error: 19.49 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 36.2 Å2 / Biso mean: 6.8841 Å2 / Biso min: 2 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.8→23.483 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 30
|
 Movie
Movie Controller
Controller













 PDBj
PDBj








