[English] 日本語
 Yorodumi
Yorodumi- PDB-5cgc: Structure of the human class C GPCR metabotropic glutamate recept... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5cgc | ||||||
|---|---|---|---|---|---|---|---|
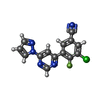
| Title | Structure of the human class C GPCR metabotropic glutamate receptor 5 transmembrane domain in complex with the negative allosteric modulator 3-chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl)pyrimidin-4-yl]benzonitrile | ||||||
 Components Components | Metabotropic glutamate receptor 5,Endolysin,Metabotropic glutamate receptor 5 | ||||||
 Keywords Keywords | SIGNALING PROTEIN / 7TM / Receptor / GPCR / Membrane-Protein | ||||||
| Function / homology |  Function and homology information Function and homology informationA2A adenosine receptor binding / neurotransmitter receptor activity involved in regulation of postsynaptic cytosolic calcium ion concentration / G protein-coupled receptor activity involved in regulation of postsynaptic membrane potential / adenylate cyclase inhibiting G protein-coupled glutamate receptor activity / phospholipase C-activating G protein-coupled glutamate receptor signaling pathway / positive regulation of long-term neuronal synaptic plasticity / desensitization of G protein-coupled receptor signaling pathway / G protein-coupled glutamate receptor signaling pathway / Class C/3 (Metabotropic glutamate/pheromone receptors) / glutamate receptor activity ...A2A adenosine receptor binding / neurotransmitter receptor activity involved in regulation of postsynaptic cytosolic calcium ion concentration / G protein-coupled receptor activity involved in regulation of postsynaptic membrane potential / adenylate cyclase inhibiting G protein-coupled glutamate receptor activity / phospholipase C-activating G protein-coupled glutamate receptor signaling pathway / positive regulation of long-term neuronal synaptic plasticity / desensitization of G protein-coupled receptor signaling pathway / G protein-coupled glutamate receptor signaling pathway / Class C/3 (Metabotropic glutamate/pheromone receptors) / glutamate receptor activity / Neurexins and neuroligins / astrocyte projection / protein tyrosine kinase activator activity / regulation of synaptic transmission, glutamatergic / viral release from host cell by cytolysis / peptidoglycan catabolic process / positive regulation of calcium-mediated signaling / protein tyrosine kinase binding / dendritic shaft / learning / locomotory behavior / G protein-coupled receptor activity / synapse organization / postsynaptic density membrane / Schaffer collateral - CA1 synapse / cognition / cellular response to amyloid-beta / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / chemical synaptic transmission / G alpha (q) signalling events / dendritic spine / host cell cytoplasm / learning or memory / positive regulation of MAPK cascade / defense response to bacterium / neuronal cell body / dendrite / regulation of DNA-templated transcription / glutamatergic synapse / identical protein binding / plasma membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) Enterobacteria phage T4 (virus) Enterobacteria phage T4 (virus) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.101 Å MOLECULAR REPLACEMENT / Resolution: 3.101 Å | ||||||
 Authors Authors | Christopher, J.A. / Aves, S.J. / Bennett, K.A. / Dore, A.S. / Errey, J.C. / Jazayeri, A. / Marshall, F.H. / Okrasa, K. / Serrano-Vega, M.J. / Tehan, B.G. ...Christopher, J.A. / Aves, S.J. / Bennett, K.A. / Dore, A.S. / Errey, J.C. / Jazayeri, A. / Marshall, F.H. / Okrasa, K. / Serrano-Vega, M.J. / Tehan, B.G. / Wiggin, G.R. / Congreve, M. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2015 Journal: J.Med.Chem. / Year: 2015Title: Fragment and Structure-Based Drug Discovery for a Class C GPCR: Discovery of the mGlu5 Negative Allosteric Modulator HTL14242 (3-Chloro-5-[6-(5-fluoropyridin-2-yl)pyrimidin-4-yl]benzonitrile). Authors: Christopher, J.A. / Aves, S.J. / Bennett, K.A. / Dore, A.S. / Errey, J.C. / Jazayeri, A. / Marshall, F.H. / Okrasa, K. / Serrano-Vega, M.J. / Tehan, B.G. / Wiggin, G.R. / Congreve, M. #1:  Journal: Nature / Year: 2014 Journal: Nature / Year: 2014Title: Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Authors: Dore, A.S. / Okrasa, K. / Patel, J.C. / Serrano-Vega, M. / Bennett, K. / Cooke, R.M. / Errey, J.C. / Jazayeri, A. / Khan, S. / Tehan, B. / Weir, M. / Wiggin, G.R. / Marshall, F.H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5cgc.cif.gz 5cgc.cif.gz | 100.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5cgc.ent.gz pdb5cgc.ent.gz | 73.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5cgc.json.gz 5cgc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5cgc_validation.pdf.gz 5cgc_validation.pdf.gz | 1.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5cgc_full_validation.pdf.gz 5cgc_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  5cgc_validation.xml.gz 5cgc_validation.xml.gz | 17.7 KB | Display | |
| Data in CIF |  5cgc_validation.cif.gz 5cgc_validation.cif.gz | 23.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cg/5cgc https://data.pdbj.org/pub/pdb/validation_reports/cg/5cgc ftp://data.pdbj.org/pub/pdb/validation_reports/cg/5cgc ftp://data.pdbj.org/pub/pdb/validation_reports/cg/5cgc | HTTPS FTP |
-Related structure data
| Related structure data |  5cgdC  4oo9S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 49802.531 Da / Num. of mol.: 1 Mutation: E579A N667Y I669A G675M C1054T C1097A T742A S753A,E579A N667Y I669A G675M C1054T C1097A T742A S753A,E579A N667Y I669A G675M C1054T C1097A T742A S753A Source method: isolated from a genetically manipulated source Details: A chimera of the human mGluR5 gene and T4-Lysozyme from enterobacteria phage T4 in complex with a new chemical entity (NCE): 3-chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl)pyrimidin-4-yl]benzonitrile Source: (gene. exp.)  Homo sapiens (human), (gene. exp.) Homo sapiens (human), (gene. exp.)  Enterobacteria phage T4 (virus) Enterobacteria phage T4 (virus)Gene: GRM5, GPRC1E, MGLUR5 / Cell line (production host): Sf21 / Production host:  | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #2: Chemical | ChemComp-OLA / #3: Chemical | ChemComp-MES / | #4: Chemical | ChemComp-51D / | #5: Water | ChemComp-HOH / | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.53 Å3/Da / Density % sol: 51.37 % |
|---|---|
| Crystal grow | Temperature: 293.1 K / Method: lipidic cubic phase / pH: 6.8 Details: 24-34% V/V PEG400, 0.2 M AMMONIUM PHOSPHATE DIBASIC, 0.1 M MES, PH 6.8, Temp details: Constant |
-Data collection
| Diffraction | Mean temperature: 200 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I24 / Wavelength: 0.96861 Å / Beamline: I24 / Wavelength: 0.96861 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: May 22, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.96861 Å / Relative weight: 1 |
| Reflection | Resolution: 3.1→32.8 Å / Num. obs: 8350 / % possible obs: 91.4 % / Redundancy: 3 % / Biso Wilson estimate: 49.91 Å2 / Rmerge(I) obs: 0.194 / Net I/σ(I): 7 |
| Reflection shell | Resolution: 3.1→3.31 Å / Redundancy: 3 % / Rmerge(I) obs: 0.522 / Mean I/σ(I) obs: 2.4 / % possible all: 93.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4OO9 Resolution: 3.101→29.735 Å / SU ML: 0.37 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 29.86 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 48.3 Å2 | ||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.101→29.735 Å
| ||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj