[English] 日本語
 Yorodumi
Yorodumi- PDB-5c52: Probing the Structural and Molecular Basis of Nucleotide Selectiv... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5c52 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Probing the Structural and Molecular Basis of Nucleotide Selectivity by Human Mitochondrial DNA Polymerase gamma | ||||||
 Components Components |
| ||||||
 Keywords Keywords | transferase/DNA / Nucleoside reverse transcriptase inhibitors (NRTIs) / HIV reverse transcriptase (RT) / human mitochondrial DNA polymerase / transferase-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationgamma DNA polymerase complex / mitochondrial chromosome / Strand-asynchronous mitochondrial DNA replication / mitochondrial DNA replication / positive regulation of DNA-directed DNA polymerase activity / DNA replication proofreading / single-stranded DNA 3'-5' DNA exonuclease activity / Hydrolases; Acting on ester bonds; Exodeoxyribonucleases producing 5'-phosphomonoesters / DNA metabolic process / DNA polymerase processivity factor activity ...gamma DNA polymerase complex / mitochondrial chromosome / Strand-asynchronous mitochondrial DNA replication / mitochondrial DNA replication / positive regulation of DNA-directed DNA polymerase activity / DNA replication proofreading / single-stranded DNA 3'-5' DNA exonuclease activity / Hydrolases; Acting on ester bonds; Exodeoxyribonucleases producing 5'-phosphomonoesters / DNA metabolic process / DNA polymerase processivity factor activity / Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases / mitochondrial nucleoid / 5'-deoxyribose-5-phosphate lyase activity / base-excision repair, gap-filling / DNA polymerase binding / 3'-5' exonuclease activity / Transcriptional activation of mitochondrial biogenesis / base-excision repair / DNA-templated DNA replication / protease binding / double-stranded DNA binding / DNA-directed DNA polymerase / in utero embryonic development / DNA-directed DNA polymerase activity / mitochondrial matrix / intracellular membrane-bounded organelle / chromatin binding / protein-containing complex / mitochondrion / DNA binding / identical protein binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)DNA launch vector pDE-GFP2 (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 3.637 Å SYNCHROTRON / Resolution: 3.637 Å | ||||||
 Authors Authors | Sohl, C.D. / Szymanski, M.R. / Mislak, A.C. / Shumate, C.K. / Amiralaei, S. / Schinazi, R.F. / Anderson, K.S. / Yin, Y.W. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2015 Journal: Proc.Natl.Acad.Sci.USA / Year: 2015Title: Probing the structural and molecular basis of nucleotide selectivity by human mitochondrial DNA polymerase gamma. Authors: Sohl, C.D. / Szymanski, M.R. / Mislak, A.C. / Shumate, C.K. / Amiralaei, S. / Schinazi, R.F. / Anderson, K.S. / Yin, Y.W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5c52.cif.gz 5c52.cif.gz | 390.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5c52.ent.gz pdb5c52.ent.gz | 300.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5c52.json.gz 5c52.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c5/5c52 https://data.pdbj.org/pub/pdb/validation_reports/c5/5c52 ftp://data.pdbj.org/pub/pdb/validation_reports/c5/5c52 ftp://data.pdbj.org/pub/pdb/validation_reports/c5/5c52 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-DNA polymerase subunit gamma- ... , 2 types, 3 molecules ABC
| #1: Protein | Mass: 135989.500 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: POLG, MDP1, POLG1, POLGA Homo sapiens (human) / Gene: POLG, MDP1, POLG1, POLGAProduction host: Insect cell expression vector pTIE1 (others) References: UniProt: P54098, DNA-directed DNA polymerase |
|---|---|
| #2: Protein | Mass: 54975.043 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: POLG2, MTPOLB Homo sapiens (human) / Gene: POLG2, MTPOLBProduction host: Insect cell expression vector pTIE1 (others) References: UniProt: Q9UHN1, DNA-directed DNA polymerase |
-DNA chain , 2 types, 2 molecules TP
| #3: DNA chain | Mass: 7955.113 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) DNA launch vector pDE-GFP2 (others) |
|---|---|
| #4: DNA chain | Mass: 6779.408 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) DNA launch vector pDE-GFP2 (others) |
-Non-polymers , 2 types, 3 molecules 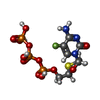


| #5: Chemical | ChemComp-1RY / [[( |
|---|---|
| #6: Chemical |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.86 Å3/Da / Density % sol: 68.16 % |
|---|---|
| Crystal grow | Temperature: 300 K / Method: vapor diffusion, sitting drop / pH: 6.5 / Details: PEG4000, KCl, HEPES |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 1 Å / Beamline: 19-ID / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Aug 1, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 3.56→51.213 Å / Num. obs: 47896 / % possible obs: 97 % / Redundancy: 9.5 % / Net I/σ(I): 2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 3.637→51.213 Å / SU ML: 0.6 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 41.07 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.637→51.213 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj









































