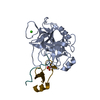
Entry Database : PDB / ID : 5bt9Title Crystal Structure of FolM Alternative dihydrofolate reductase 1 from Brucella canis complexed with NADP 3-oxoacyl-(Acyl-carrier-protein) reductase Keywords / / / / / / Function / homology Function Domain/homology Component
/ / / / / / Biological species Brucella canis Method / / / / Resolution : 1.5 Å Authors Seattle Structural Genomics Center for Infectious Disease (SSGCID) Journal : Acta Crystallogr.,Sect.F / Year : 2022Title : Crystal structures of FolM alternative dihydrofolate reductase 1 from Brucella suis and Brucella canis.Authors: Porter, I. / Neal, T. / Walker, Z. / Hayes, D. / Fowler, K. / Billups, N. / Rhoades, A. / Smith, C. / Smith, K. / Staker, B.L. / Dranow, D.M. / Mayclin, S.J. / Subramanian, S. / Edwards, T.E. ... Authors : Porter, I. / Neal, T. / Walker, Z. / Hayes, D. / Fowler, K. / Billups, N. / Rhoades, A. / Smith, C. / Smith, K. / Staker, B.L. / Dranow, D.M. / Mayclin, S.J. / Subramanian, S. / Edwards, T.E. / Myler, P.J. / Asojo, O.A. History Deposition Jun 2, 2015 Deposition site / Processing site Revision 1.0 Jul 29, 2015 Provider / Type Revision 1.1 Oct 7, 2015 Group / Source and taxonomyRevision 1.2 Sep 20, 2023 Group / Database references / Derived calculationsCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / citation / citation_author / database_2 / pdbx_struct_oper_list Item _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_ASTM / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_oper_list.symmetry_operation Revision 1.3 Sep 27, 2023 Group / Category Revision 1.4 Oct 22, 2025 Group / Category / pdbx_entry_details
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.5 Å
molecular replacement / Resolution: 1.5 Å  Authors
Authors Citation
Citation Journal: Acta Crystallogr.,Sect.F / Year: 2022
Journal: Acta Crystallogr.,Sect.F / Year: 2022 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5bt9.cif.gz
5bt9.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5bt9.ent.gz
pdb5bt9.ent.gz PDB format
PDB format 5bt9.json.gz
5bt9.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/bt/5bt9
https://data.pdbj.org/pub/pdb/validation_reports/bt/5bt9 ftp://data.pdbj.org/pub/pdb/validation_reports/bt/5bt9
ftp://data.pdbj.org/pub/pdb/validation_reports/bt/5bt9

 Links
Links Assembly
Assembly




 Components
Components Brucella canis (strain ATCC 23365 / NCTC 10854) (bacteria)
Brucella canis (strain ATCC 23365 / NCTC 10854) (bacteria)
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 21-ID-F / Wavelength: 0.97872 Å
/ Beamline: 21-ID-F / Wavelength: 0.97872 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller











 PDBj
PDBj



