+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4zjm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
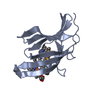
| Title | Crystal Structure of Mycobacterium tuberculosis LpqH (Rv3763) | ||||||||||||
 Components Components | (Lipoprotein LpqH) x 7 | ||||||||||||
 Keywords Keywords | UNKNOWN FUNCTION / lipoprotein / virulence / structural genomics / PSI-2 / Protein Structure Initiative / TB Structural Genomics Consortium / TBSGC | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host immune response / bacterial extracellular vesicle / biological process involved in interaction with host / peptidoglycan-based cell wall / host cell cytoplasm / defense response to bacterium / host cell surface receptor binding / cell surface / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.851 Å MOLECULAR REPLACEMENT / Resolution: 2.851 Å | ||||||||||||
 Authors Authors | Arbing, M.A. / Chan, S. / Kuo, E. / Harris, L.R. / Zhou, T.T. / Eisenberg, D. / TB Structural Genomics Consortium (TBSGC) | ||||||||||||
| Funding support |  United States, 3items United States, 3items
| ||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Crystal Structure of Mycobacterium tuberculosis LpqH (Rv3763) Authors: Arbing, M.A. / Chan, S. / Kuo, E. / Harris, L.R. / Zhou, T.T. / Eisenberg, D. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4zjm.cif.gz 4zjm.cif.gz | 290.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4zjm.ent.gz pdb4zjm.ent.gz | 238.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4zjm.json.gz 4zjm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zj/4zjm https://data.pdbj.org/pub/pdb/validation_reports/zj/4zjm ftp://data.pdbj.org/pub/pdb/validation_reports/zj/4zjm ftp://data.pdbj.org/pub/pdb/validation_reports/zj/4zjm | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4xinS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
- Components
Components
-Protein , 7 types, 7 molecules ABCDEFG
| #1: Protein | Mass: 11956.234 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)Strain: ATCC 25618 / H37Rv / Gene: lpqH, Rv3763, MTV025.111 / Plasmid: pMAPLe3 / Details (production host): pET28 derivative / Production host:  |
|---|---|
| #2: Protein | Mass: 11902.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)Strain: ATCC 25618 / H37Rv / Gene: lpqH, Rv3763, MTV025.111 / Plasmid: pMAPLe3 / Details (production host): pET28 derivative / Production host: Escherichia coli / Strain (production host): BL21 / Variant (production host): (DE3) / References: UniProt: P9WK61 |
| #3: Protein | Mass: 11875.097 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)Strain: ATCC 25618 / H37Rv / Gene: lpqH, Rv3763, MTV025.111 / Plasmid: pMAPLe3 / Details (production host): pET28 derivative / Production host: Escherichia coli / Strain (production host): BL21 / Variant (production host): (DE3) / References: UniProt: P9WK61 |
| #4: Protein | Mass: 11875.097 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)Strain: ATCC 25618 / H37Rv / Gene: lpqH, Rv3763, MTV025.111 / Plasmid: pMAPLe3 / Details (production host): pET28 derivative / Production host: Escherichia coli / Strain (production host): BL21 / Variant (production host): (DE3) / References: UniProt: P9WK61 |
| #5: Protein | Mass: 11902.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)Strain: ATCC 25618 / H37Rv / Gene: lpqH, Rv3763, MTV025.111 / Plasmid: pMAPLe3 / Details (production host): pET28 derivative / Production host: Escherichia coli / Strain (production host): BL21 / Variant (production host): (DE3) / References: UniProt: P9WK61 |
| #6: Protein | Mass: 11902.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)Strain: ATCC 25618 / H37Rv / Gene: lpqH, Rv3763, MTV025.111 / Plasmid: pMAPLe3 / Details (production host): pET28 derivative / Production host: Escherichia coli / Strain (production host): BL21 / Variant (production host): (DE3) / References: UniProt: P9WK61 |
| #7: Protein | Mass: 11929.189 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria) Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)Strain: ATCC 25618 / H37Rv / Gene: lpqH, Rv3763, MTV025.111 / Plasmid: pMAPLe3 / Details (production host): pET28 derivative / Production host: Escherichia coli / Strain (production host): BL21 / Variant (production host): (DE3) / References: UniProt: P9WK61 |
-Non-polymers , 4 types, 39 molecules 






| #8: Chemical | ChemComp-SO4 / #9: Chemical | #10: Chemical | ChemComp-CL / | #11: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.63 Å3/Da / Density % sol: 66.13 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 2:1 ratio of protein to reservoir solution (2 M ammonium sulfate). 200 nanoliter drop size. Protein buffer: 50 mM HEPES, pH 7.8, 150 mM NaCl. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.9794 Å / Beamline: 24-ID-C / Wavelength: 0.9794 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jul 25, 2011 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9794 Å / Relative weight: 1 |
| Reflection | Resolution: 2.85→19.832 Å / Num. obs: 29240 / % possible obs: 99.87 % / Observed criterion σ(I): -3 / Redundancy: 6.8 % / Biso Wilson estimate: 69.23 Å2 / Rmerge(I) obs: 0.1181 / Net I/σ(I): 15.22 |
| Reflection shell | Resolution: 2.85→2.93 Å / Redundancy: 6.8 % / Rmerge(I) obs: 1.494 / Mean I/σ(I) obs: 1.88 / % possible all: 98.96 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4XIN Resolution: 2.851→19.832 Å / SU ML: 0.4 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 28.83 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 86.4885 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.851→19.832 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj















