Entry Database : PDB / ID : 4xojTitle Structure of bovine trypsin in complex with analogues of sunflower inhibitor 1 (SFTI-1) Cationic trypsin Trypsin inhibitor 1 Keywords / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Bos taurus (domestic cattle)Helianthus annuus (common sunflower)Method / / / Resolution : 0.91 Å Authors Golik, P. / Malicki, S. / Grudnik, P. / Karna, N. / Debowski, D. / Legowska, A. / Wladyka, B. / Gitlin, A. / Brzozowski, K. / Dubin, G. / Rolka, K. Journal : Chembiochem / Year : 2015Title : Investigation of Serine-Proteinase-Catalyzed Peptide Splicing in Analogues of Sunflower Trypsin Inhibitor 1 (SFTI-1).Authors : Karna, N. / Legowska, A. / Malicki, S. / Debowski, D. / Golik, P. / Gitlin, A. / Grudnik, P. / Wladyka, B. / Brzozowski, K. / Dubin, G. / Rolka, K. History Deposition Jan 16, 2015 Deposition site / Processing site Revision 1.0 Aug 12, 2015 Provider / Type Revision 1.1 Sep 23, 2015 Group Revision 1.2 Aug 9, 2017 Group / Database references / Category / diffrn_sourceItem / _diffrn_source.pdbx_synchrotron_siteRevision 1.3 Jan 10, 2024 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_asym_id / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_alt_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_asym_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_alt_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_ptnr2_label_alt_id / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id Revision 1.4 Oct 9, 2024 Group / Category / pdbx_modification_feature
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
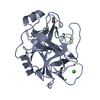
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information

 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 0.91 Å
MOLECULAR REPLACEMENT / Resolution: 0.91 Å  Authors
Authors Citation
Citation Journal: Chembiochem / Year: 2015
Journal: Chembiochem / Year: 2015 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4xoj.cif.gz
4xoj.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4xoj.ent.gz
pdb4xoj.ent.gz PDB format
PDB format 4xoj.json.gz
4xoj.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/xo/4xoj
https://data.pdbj.org/pub/pdb/validation_reports/xo/4xoj ftp://data.pdbj.org/pub/pdb/validation_reports/xo/4xoj
ftp://data.pdbj.org/pub/pdb/validation_reports/xo/4xoj
 Links
Links Assembly
Assembly
 Components
Components












 X-RAY DIFFRACTION
X-RAY DIFFRACTION Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  PETRA III, EMBL c/o DESY
PETRA III, EMBL c/o DESY  / Beamline: P13 (MX1) / Wavelength: 1 Å
/ Beamline: P13 (MX1) / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller











 PDBj
PDBj






