[English] 日本語
 Yorodumi
Yorodumi- PDB-4v1l: High resolution structure of a novel carbohydrate binding module ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4v1l | ||||||
|---|---|---|---|---|---|---|---|
| Title | High resolution structure of a novel carbohydrate binding module from glycoside hydrolase family 9 (Cel9A) from Ruminococcus flavefaciens FD-1 | ||||||
 Components Components | CARBOHYDRATE BINDING MODULE | ||||||
 Keywords Keywords | SUGAR BINDING PROTEIN / CEL9A / CELLULOSOME | ||||||
| Function / homology | Domain of unknown function DUF5620 / Domain of unknown function (DUF5620) / TRIETHYLENE GLYCOL / Carbohydrate binding module Function and homology information Function and homology information | ||||||
| Biological species |  RUMINOCOCCUS FLAVEFACIENS (bacteria) RUMINOCOCCUS FLAVEFACIENS (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.75 Å MOLECULAR REPLACEMENT / Resolution: 1.75 Å | ||||||
 Authors Authors | Venditto, I. / Goyal, A. / Thompson, A. / Ferreira, L.M.A. / Fontes, C.M.G.A. / Najmudin, S. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2016 Journal: Proc.Natl.Acad.Sci.USA / Year: 2016Title: Complexity of the Ruminococcus Flavefaciens Cellulosome Reflects an Expansion in Glycan Recognition. Authors: Venditto, I. / Luis, A.S. / Rydahl, M. / Schuckel, J. / Fernandes, V.O. / Vidal-Melgosa, S. / Bule, P. / Goyal, A. / Pires, V.M.R. / Dourado, C.G. / Ferreira, L.M.A. / Coutinho, P.M. / ...Authors: Venditto, I. / Luis, A.S. / Rydahl, M. / Schuckel, J. / Fernandes, V.O. / Vidal-Melgosa, S. / Bule, P. / Goyal, A. / Pires, V.M.R. / Dourado, C.G. / Ferreira, L.M.A. / Coutinho, P.M. / Henrissat, B. / Knox, J.P. / Basle, A. / Najmudin, S. / Gilbert, H.J. / Willats, W.G.T. / Fontes, C.M.G.A. #1: Journal: Acta Crystallogr F Struct Biol Commun / Year: 2015 Title: Crystallization and preliminary crystallographic studies of a novel noncatalytic carbohydrate-binding module from the Ruminococcus flavefaciens cellulosome. Authors: Venditto, I. / Goyal, A. / Thompson, A. / Ferreira, L.M. / Fontes, C.M. / Najmudin, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4v1l.cif.gz 4v1l.cif.gz | 177.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4v1l.ent.gz pdb4v1l.ent.gz | 142.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4v1l.json.gz 4v1l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/v1/4v1l https://data.pdbj.org/pub/pdb/validation_reports/v1/4v1l ftp://data.pdbj.org/pub/pdb/validation_reports/v1/4v1l ftp://data.pdbj.org/pub/pdb/validation_reports/v1/4v1l | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4d3lC  4v17C  4v18C  4v1bC  4v1iC  4v1kSC  5aosC  5aotC  5fu2C  5fu3C  5fu4C  5fu5C C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 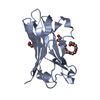
| ||||||||||||||||||
| 2 | 
| ||||||||||||||||||
| 3 | 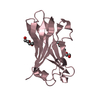
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 15329.945 Da / Num. of mol.: 3 / Fragment: CARBOHYDRATE BINDING MODULE, RESIDUES 492-629 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  RUMINOCOCCUS FLAVEFACIENS (bacteria) / Strain: FD-1 / Plasmid: PET-28A / Production host: RUMINOCOCCUS FLAVEFACIENS (bacteria) / Strain: FD-1 / Plasmid: PET-28A / Production host:  |
|---|
-Non-polymers , 5 types, 360 molecules 








| #2: Chemical | | #3: Chemical | #4: Chemical | #5: Chemical | ChemComp-PG4 / | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Nonpolymer details | TETRAETHYLENE GLYCOL (PG4): JUST BASED ON ELECTRON DENSITY AND IS PROBABLY AN ARTEFACT FROM THE ...TETRAETHYL |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.17 Å3/Da / Density % sol: 61 % / Description: NONE |
|---|---|
| Crystal grow | pH: 6.5 Details: 1 M SODIUM CITRATE, 0.1 M MES PH 6.5, 30% GLYCEROL WAS ADDED IN ABOVE CONDITOIN FOR TEH CRYOPROTECTANT |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM30A / Wavelength: 0.9792 / Beamline: BM30A / Wavelength: 0.9792 |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Oct 31, 2013 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9792 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→30.19 Å / Num. obs: 58166 / % possible obs: 100 % / Observed criterion σ(I): 0 / Redundancy: 13.9 % / Rmerge(I) obs: 0.13 / Net I/σ(I): 15.8 |
| Reflection shell | Resolution: 1.75→1.84 Å / Redundancy: 11.3 % / Rmerge(I) obs: 1.38 / Mean I/σ(I) obs: 1.8 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4V1K Resolution: 1.75→74.83 Å / Cor.coef. Fo:Fc: 0.969 / Cor.coef. Fo:Fc free: 0.964 / SU B: 3.848 / SU ML: 0.06 / Cross valid method: THROUGHOUT / ESU R: 0.084 / ESU R Free: 0.082 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES WITH TLS ADDED. LIGAND IDENTIFIER FROM PHENIX SUITE AND PDB_REDO WERE USED DURING REFINEMENT. DISORDERED REGIONS WERE MODELED STEREOCHEMICALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 26.966 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.75→74.83 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj


