[English] 日本語
 Yorodumi
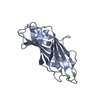
Yorodumi- PDB-4txq: Crystal structure of LIP5 N-terminal domain complexed with CHMP1B MIM -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4txq | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of LIP5 N-terminal domain complexed with CHMP1B MIM | ||||||
 Components Components |
| ||||||
 Keywords Keywords | PROTEIN TRANSPORT / MIT / MIT domain / ESCRT | ||||||
| Function / homology |  Function and homology information Function and homology informationMIT domain binding / multivesicular body-lysosome fusion / amphisome membrane / vesicle fusion with vacuole / ESCRT III complex disassembly / late endosome to lysosome transport / ESCRT III complex / kinetochore microtubule / endosome transport via multivesicular body sorting pathway / late endosome to vacuole transport via multivesicular body sorting pathway ...MIT domain binding / multivesicular body-lysosome fusion / amphisome membrane / vesicle fusion with vacuole / ESCRT III complex disassembly / late endosome to lysosome transport / ESCRT III complex / kinetochore microtubule / endosome transport via multivesicular body sorting pathway / late endosome to vacuole transport via multivesicular body sorting pathway / membrane coat / nuclear membrane reassembly / multivesicular body sorting pathway / regulation of centrosome duplication / midbody abscission / membrane fission / plasma membrane repair / late endosome to vacuole transport / ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway / multivesicular body assembly / multivesicular body membrane / regulation of mitotic spindle assembly / mitotic metaphase chromosome alignment / nucleus organization / viral budding via host ESCRT complex / autophagosome membrane / autophagosome maturation / nuclear pore / multivesicular body / Endosomal Sorting Complex Required For Transport (ESCRT) / viral budding from plasma membrane / macroautophagy / establishment of protein localization / Budding and maturation of HIV virion / kinetochore / autophagy / protein transport / midbody / endosome membrane / intracellular membrane-bounded organelle / protein domain specific binding / cell division / lysosomal membrane / extracellular exosome / nucleoplasm / identical protein binding / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.21 Å MOLECULAR REPLACEMENT / Resolution: 2.21 Å | ||||||
 Authors Authors | Vild, C.J. / Xu, Z. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2015 Journal: J.Biol.Chem. / Year: 2015Title: A Novel Mechanism of Regulating the ATPase VPS4 by Its Cofactor LIP5 and the Endosomal Sorting Complex Required for Transport (ESCRT)-III Protein CHMP5. Authors: Vild, C.J. / Li, Y. / Guo, E.Z. / Liu, Y. / Xu, Z. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4txq.cif.gz 4txq.cif.gz | 88.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4txq.ent.gz pdb4txq.ent.gz | 65.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4txq.json.gz 4txq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tx/4txq https://data.pdbj.org/pub/pdb/validation_reports/tx/4txq ftp://data.pdbj.org/pub/pdb/validation_reports/tx/4txq ftp://data.pdbj.org/pub/pdb/validation_reports/tx/4txq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4txpSC  4txrC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 18792.727 Da / Num. of mol.: 2 / Fragment: UNP residues 1-162 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: VTA1, C6orf55, HSPC228, My012 / Plasmid: pSMT3 Homo sapiens (human) / Gene: VTA1, C6orf55, HSPC228, My012 / Plasmid: pSMT3Details (production host): modified pET28b; his6-sumo tag; kanR Production host:  #2: Protein/peptide | Mass: 2718.953 Da / Num. of mol.: 2 / Fragment: UNP residues 176-199 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CHMP1B, C18orf2 / Plasmid: pSMT3 Homo sapiens (human) / Gene: CHMP1B, C18orf2 / Plasmid: pSMT3Details (production host): modified pET28b; his6-sumo tag; kanR Production host:  #3: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.34 Å3/Da / Density % sol: 63.13 % |
|---|---|
| Crystal grow | Temperature: 277.15 K / Method: vapor diffusion, sitting drop / pH: 9 Details: Protein mixture was added in 1:1 ratio with a solution of 16% MPD, 0.1 M Tris and equilibrated against a mother liquid of 8% MPD, 0.1 M Tris |
-Data collection
| Diffraction | Mean temperature: 193.15 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-D / Wavelength: 0.97872 Å / Beamline: 21-ID-D / Wavelength: 0.97872 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Dec 14, 2012 |
| Radiation | Monochromator: diamond laue monochromators with beryllium lenses Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97872 Å / Relative weight: 1 |
| Reflection | Resolution: 2.208→41.076 Å / Num. obs: 29702 / % possible obs: 99.9 % / Redundancy: 7.3 % / Rmerge(I) obs: 0.11 / Net I/σ(I): 19.4 |
| Reflection shell | Resolution: 2.208→2.28 Å / Redundancy: 7.4 % / Rmerge(I) obs: 0.55 / Mean I/σ(I) obs: 3.2 / % possible all: 100 |
- Processing
Processing
| Software | Name: PHENIX / Version: (phenix.refine: dev_1593) / Classification: refinement | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4TXP Resolution: 2.21→41.076 Å / SU ML: 0.2 / Cross valid method: THROUGHOUT / σ(F): 1.36 / Phase error: 22.8 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.21→41.076 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj


