[English] 日本語
 Yorodumi
Yorodumi- PDB-4ph8: Crystal structure of AggA, the major subunit of aggregative adher... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4ph8 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
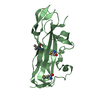
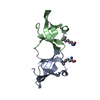
| Title | Crystal structure of AggA, the major subunit of aggregative adherence fimbriae type I (AAF/I) from the Escherichia coli O4H104 | ||||||||||||
 Components Components | Aggregative adherence fimbrial subunit AggA | ||||||||||||
 Keywords Keywords | CELL ADHESION / Beta sandwich / donor-strand complementation / fibronectin binding | ||||||||||||
| Function / homology | Immunoglobulin-like - #2910 / Immunoglobulin-like / Sandwich / Mainly Beta / :  Function and homology information Function and homology information | ||||||||||||
| Biological species |  | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 1.55 Å SAD / Resolution: 1.55 Å | ||||||||||||
| Model details | structure is linked with 4OR1 | ||||||||||||
 Authors Authors | Pakharukova, N.A. / Tuitilla, M. / Zavialov, A.V. | ||||||||||||
| Funding support |  Finland, 3items Finland, 3items
| ||||||||||||
 Citation Citation |  Journal: Plos Pathog. / Year: 2014 Journal: Plos Pathog. / Year: 2014Title: Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative Escherichia coli. Authors: Berry, A.A. / Yang, Y. / Pakharukova, N. / Garnett, J.A. / Lee, W.C. / Cota, E. / Marchant, J. / Roy, S. / Tuittila, M. / Liu, B. / Inman, K.G. / Ruiz-Perez, F. / Mandomando, I. / Nataro, J. ...Authors: Berry, A.A. / Yang, Y. / Pakharukova, N. / Garnett, J.A. / Lee, W.C. / Cota, E. / Marchant, J. / Roy, S. / Tuittila, M. / Liu, B. / Inman, K.G. / Ruiz-Perez, F. / Mandomando, I. / Nataro, J.P. / Zavialov, A.V. / Matthews, S. #1: Journal: Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. Year: 2013 Title: Crystallization and sulfur SAD phasing of AggA, the major subunit of aggregative adherence fimbriae type I from the Escherichia coli strain that caused an outbreak of haemolytic-uraemic syndrome in Germany. Authors: Pakharukova, N. / Tuittila, M. / Zavialov, A. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4ph8.cif.gz 4ph8.cif.gz | 139.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4ph8.ent.gz pdb4ph8.ent.gz | 109.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4ph8.json.gz 4ph8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4ph8_validation.pdf.gz 4ph8_validation.pdf.gz | 453.4 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4ph8_full_validation.pdf.gz 4ph8_full_validation.pdf.gz | 457.1 KB | Display | |
| Data in XML |  4ph8_validation.xml.gz 4ph8_validation.xml.gz | 16.3 KB | Display | |
| Data in CIF |  4ph8_validation.cif.gz 4ph8_validation.cif.gz | 23.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ph/4ph8 https://data.pdbj.org/pub/pdb/validation_reports/ph/4ph8 ftp://data.pdbj.org/pub/pdb/validation_reports/ph/4ph8 ftp://data.pdbj.org/pub/pdb/validation_reports/ph/4ph8 | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | biological unit is a fimbriae made of polymerised of AggA subunits, and on the tip of the fimbriae minor subunit (AggB) is located. Assymmetric unit consists of two AggA subunits |
- Components
Components
| #1: Protein | Mass: 16768.891 Da / Num. of mol.: 2 / Fragment: Residues 41-167 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: EUAG_05069 / Plasmid: pET101D / Production host:  #2: Chemical | #3: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.22 Å3/Da / Density % sol: 44.56 % / Description: Single crystal, dimensions 0.6 mm x 0.2 mm |
|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 0.2M sodium acetate, 0.1M sodium cacodylate, 30% w/v PEG 8000 |
-Data collection
| Diffraction |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.55→91.416 Å / Num. all: 40475 / Num. obs: 40475 / % possible obs: 97.2 % / Redundancy: 4.2 % / Rpim(I) all: 0.021 / Rrim(I) all: 0.044 / Rsym value: 0.038 / Net I/av σ(I): 14.273 / Net I/σ(I): 19 / Num. measured all: 170118 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
-Phasing
| Phasing | Method:  SAD SAD |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 1.55→55.84 Å / Cor.coef. Fo:Fc: 0.968 / Cor.coef. Fo:Fc free: 0.933 / WRfactor Rfree: 0.2224 / WRfactor Rwork: 0.1503 / FOM work R set: 0.8261 / SU B: 4.706 / SU ML: 0.073 / SU R Cruickshank DPI: 0.1125 / SU Rfree: 0.1005 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.113 / ESU R Free: 0.101 / Stereochemistry target values: MAXIMUM LIKELIHOOD SAD / Resolution: 1.55→55.84 Å / Cor.coef. Fo:Fc: 0.968 / Cor.coef. Fo:Fc free: 0.933 / WRfactor Rfree: 0.2224 / WRfactor Rwork: 0.1503 / FOM work R set: 0.8261 / SU B: 4.706 / SU ML: 0.073 / SU R Cruickshank DPI: 0.1125 / SU Rfree: 0.1005 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.113 / ESU R Free: 0.101 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 84.87 Å2 / Biso mean: 24.834 Å2 / Biso min: 8.53 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.55→55.84 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.55→1.59 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller













 PDBj
PDBj



