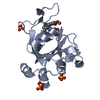
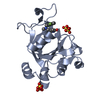
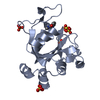
Entry Database : PDB / ID : 4oy8Title Structure of ScLPMO10B in complex with zinc. Putative secreted cellulose-binding protein Keywords / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / Biological species Streptomyces coelicolor (bacteria)Method / / Resolution : 1.4 Å Authors Forsberg, Z. / Mackenzie, A.K. / Sorlie, M. / Rohr, A.K. / Helland, R. / Arvai, A.S. / Vaaje-Kolstad, G. / Eijsink, V.G.H. Journal : Proc.Natl.Acad.Sci.USA / Year : 2014Title : Structural and functional characterization of a conserved pair of bacterial cellulose-oxidizing lytic polysaccharide monooxygenases.Authors : Forsberg, Z. / Mackenzie, A.K. / Srlie, M. / Rhr, A.K. / Helland, R. / Arvai, A.S. / Vaaje-Kolstad, G. / Eijsink, V.G. History Deposition Feb 11, 2014 Deposition site / Processing site Revision 1.0 May 28, 2014 Provider / Type Revision 1.1 Jun 25, 2014 Group Revision 1.2 Feb 4, 2015 Group Revision 1.3 Sep 27, 2017 Group Author supporting evidence / Database references ... Author supporting evidence / Database references / Derived calculations / Other / Source and taxonomy / Structure summary Category citation / entity_src_gen ... citation / entity_src_gen / pdbx_audit_support / pdbx_database_status / pdbx_struct_assembly / pdbx_struct_conn_angle / pdbx_struct_oper_list / struct_keywords / symmetry Item _citation.journal_id_CSD / _entity_src_gen.pdbx_alt_source_flag ... _citation.journal_id_CSD / _entity_src_gen.pdbx_alt_source_flag / _pdbx_database_status.pdb_format_compatible / _pdbx_struct_assembly.oligomeric_details / _pdbx_struct_oper_list.symmetry_operation / _struct_keywords.text / _symmetry.Int_Tables_number Revision 1.4 Dec 27, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_struct_conn_angle / refine_hist / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr1_symmetry / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr2_symmetry / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.ptnr3_symmetry / _pdbx_struct_conn_angle.value / _refine_hist.number_atoms_solvent / _refine_hist.number_atoms_total / _refine_hist.pdbx_number_atoms_ligand / _refine_hist.pdbx_number_atoms_nucleic_acid / _refine_hist.pdbx_number_atoms_protein / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_symmetry Revision 1.5 Oct 23, 2024 Group / Category / pdbx_modification_feature
Show all Show less Remark 650 HELIX DETERMINATION METHOD: AUTHOR PROVIDED.
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Streptomyces coelicolor (bacteria)
Streptomyces coelicolor (bacteria) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.4 Å
SYNCHROTRON / Resolution: 1.4 Å  Authors
Authors Citation
Citation Journal: Proc.Natl.Acad.Sci.USA / Year: 2014
Journal: Proc.Natl.Acad.Sci.USA / Year: 2014 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4oy8.cif.gz
4oy8.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4oy8.ent.gz
pdb4oy8.ent.gz PDB format
PDB format 4oy8.json.gz
4oy8.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/oy/4oy8
https://data.pdbj.org/pub/pdb/validation_reports/oy/4oy8 ftp://data.pdbj.org/pub/pdb/validation_reports/oy/4oy8
ftp://data.pdbj.org/pub/pdb/validation_reports/oy/4oy8 Links
Links Assembly
Assembly
 Components
Components Streptomyces coelicolor (bacteria) / Strain: A3(2) / Gene: SCO0643 / Plasmid: pRSET B / Production host:
Streptomyces coelicolor (bacteria) / Strain: A3(2) / Gene: SCO0643 / Plasmid: pRSET B / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID14-4 / Wavelength: 1.28 Å
/ Beamline: ID14-4 / Wavelength: 1.28 Å Processing
Processing Movie
Movie Controller
Controller












 PDBj
PDBj



