| Entry | Database: PDB / ID: 4nlh
|
|---|
| Title | SKICH domain of human TAX1BP1 |
|---|
 Components Components | Tax1-binding protein 1 |
|---|
 Keywords Keywords | PROTEIN BINDING / Ig like fold |
|---|
| Function / homology |  Function and homology information Function and homology information
protein localization to phagocytic vesicle / negative regulation of interleukin-1-mediated signaling pathway / negative regulation of cytoplasmic pattern recognition receptor signaling pathway / negative regulation of toll-like receptor 4 signaling pathway / phagophore assembly site / negative regulation of tumor necrosis factor-mediated signaling pathway / signaling adaptor activity / negative regulation of canonical NF-kappaB signal transduction / autophagosome / Negative regulators of DDX58/IFIH1 signaling ...protein localization to phagocytic vesicle / negative regulation of interleukin-1-mediated signaling pathway / negative regulation of cytoplasmic pattern recognition receptor signaling pathway / negative regulation of toll-like receptor 4 signaling pathway / phagophore assembly site / negative regulation of tumor necrosis factor-mediated signaling pathway / signaling adaptor activity / negative regulation of canonical NF-kappaB signal transduction / autophagosome / Negative regulators of DDX58/IFIH1 signaling / Regulation of TNFR1 signaling / autophagy / kinase binding / cytoplasmic vesicle / protein-macromolecule adaptor activity / innate immune response / apoptotic process / negative regulation of apoptotic process / mitochondrion / extracellular exosome / zinc ion binding / cytosol / cytoplasmSimilarity search - Function Immunoglobulin-like - #2840 / Calcium binding and coiled-coil domain-like / Calcium binding and coiled-coil domain (CALCOCO1) like / Autophagy receptor zinc finger-C2H2 domain / SKICH domain / : / SKICH domain / CALCOCO1/2, zinc finger UBZ1-type / Zinc finger UBZ1-type profile. / Immunoglobulin-like ...Immunoglobulin-like - #2840 / Calcium binding and coiled-coil domain-like / Calcium binding and coiled-coil domain (CALCOCO1) like / Autophagy receptor zinc finger-C2H2 domain / SKICH domain / : / SKICH domain / CALCOCO1/2, zinc finger UBZ1-type / Zinc finger UBZ1-type profile. / Immunoglobulin-like / Sandwich / Mainly BetaSimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å |
|---|
 Authors Authors | Yang, Y. / Wang, G. / Huang, X. / Zhu, J. / Du, Z. |
|---|
 Citation Citation |  Journal: To be published Journal: To be published
Title: SKICH domain of human TAX1BP1
Authors: Yang, Y. / Wang, G. / Huang, X. / Zhu, J. / Du, Z. |
|---|
| History | | Deposition | Nov 14, 2013 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Dec 24, 2014 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Feb 28, 2024 | Group: Data collection / Database references
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / struct_ref_seq_dif
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_ref_seq_dif.details |
|---|
|
|---|
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å
MOLECULAR REPLACEMENT / Resolution: 1.9 Å  Authors
Authors Citation
Citation Journal: To be published
Journal: To be published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4nlh.cif.gz
4nlh.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4nlh.ent.gz
pdb4nlh.ent.gz PDB format
PDB format 4nlh.json.gz
4nlh.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 4nlh_validation.pdf.gz
4nlh_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 4nlh_full_validation.pdf.gz
4nlh_full_validation.pdf.gz 4nlh_validation.xml.gz
4nlh_validation.xml.gz 4nlh_validation.cif.gz
4nlh_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/nl/4nlh
https://data.pdbj.org/pub/pdb/validation_reports/nl/4nlh ftp://data.pdbj.org/pub/pdb/validation_reports/nl/4nlh
ftp://data.pdbj.org/pub/pdb/validation_reports/nl/4nlh Links
Links Assembly
Assembly
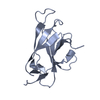

 Components
Components Homo sapiens (human) / Gene: HUMAN TAX1BP1, PRO0105, T6BP, TAX1BP1 / Production host:
Homo sapiens (human) / Gene: HUMAN TAX1BP1, PRO0105, T6BP, TAX1BP1 / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 21-ID-F / Wavelength: 0.97872 Å
/ Beamline: 21-ID-F / Wavelength: 0.97872 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 1.9→34.689 Å / Occupancy max: 1 / Occupancy min: 1 / FOM work R set: 0.7119 / SU ML: 0.39 / σ(F): 0 / Phase error: 33.41 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 1.9→34.689 Å / Occupancy max: 1 / Occupancy min: 1 / FOM work R set: 0.7119 / SU ML: 0.39 / σ(F): 0 / Phase error: 33.41 / Stereochemistry target values: ML Movie
Movie Controller
Controller













 PDBj
PDBj


