| Entry | Database: PDB / ID: 4led
|
|---|
| Title | The Crystal Structure of Pyocin L1 bound to D-rhamnose at 2.37 Angstroms |
|---|
 Components Components | Pyocin L1 |
|---|
 Keywords Keywords | SUGAR BINDING PROTEIN / Monocot Mannose Binding Protein / Galanthus nivalis agglutinin / Beta Prism / Bacteriocin / Antimicrobial Protein / Extracellular |
|---|
| Function / homology |  Function and homology information Function and homology information
Agglutinin, subunit A - #30 / Agglutinin, subunit A / Bulb-type lectin domain / Bulb-type lectin domain / Bulb-type lectin domain superfamily / Bulb-type lectin domain profile. / Bulb-type mannose-specific lectin / Orthogonal Prism / Mainly BetaSimilarity search - Domain/homology |
|---|
| Biological species |   Pseudomonas aeruginosa (bacteria) Pseudomonas aeruginosa (bacteria) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.37 Å MOLECULAR REPLACEMENT / Resolution: 2.37 Å |
|---|
 Authors Authors | Grinter, R. / Roszak, A.W. / Mccaughey, L. / Cogdell, C.J. / Walker, D. |
|---|
 Citation Citation |  Journal: Plos Pathog. / Year: 2014 Journal: Plos Pathog. / Year: 2014
Title: Lectin-Like Bacteriocins from Pseudomonas spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor.
Authors: McCaughey, L.C. / Grinter, R. / Josts, I. / Roszak, A.W. / Walen, K.I. / Cogdell, R.J. / Milner, J. / Evans, T. / Kelly, S. / Tucker, N.P. / Byron, O. / Smith, B. / Walker, D. |
|---|
| History | | Deposition | Jun 25, 2013 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Feb 19, 2014 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Mar 19, 2014 | Group: Database references |
|---|
| Revision 1.2 | Jul 29, 2020 | Group: Data collection / Derived calculations / Structure summary
Category: chem_comp / entity ...chem_comp / entity / pdbx_chem_comp_identifier / pdbx_entity_nonpoly / struct_site / struct_site_gen
Item: _chem_comp.name / _chem_comp.type ..._chem_comp.name / _chem_comp.type / _entity.pdbx_description / _pdbx_entity_nonpoly.name
Description: Carbohydrate remediation / Provider: repository / Type: Remediation |
|---|
| Revision 1.3 | Sep 20, 2023 | Group: Data collection / Database references ...Data collection / Database references / Refinement description / Structure summary
Category: chem_comp / chem_comp_atom ...chem_comp / chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model
Item: _chem_comp.pdbx_synonyms / _database_2.pdbx_DOI / _database_2.pdbx_database_accession |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.37 Å
MOLECULAR REPLACEMENT / Resolution: 2.37 Å  Authors
Authors Citation
Citation Journal: Plos Pathog. / Year: 2014
Journal: Plos Pathog. / Year: 2014 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4led.cif.gz
4led.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4led.ent.gz
pdb4led.ent.gz PDB format
PDB format 4led.json.gz
4led.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/le/4led
https://data.pdbj.org/pub/pdb/validation_reports/le/4led ftp://data.pdbj.org/pub/pdb/validation_reports/le/4led
ftp://data.pdbj.org/pub/pdb/validation_reports/le/4led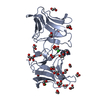

 Links
Links Assembly
Assembly


 Components
Components

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I04 / Wavelength: 0.9788 Å
/ Beamline: I04 / Wavelength: 0.9788 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller












 PDBj
PDBj