+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4gtr | ||||||
|---|---|---|---|---|---|---|---|
| Title | FTase in complex with BMS analogue 13 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR / protein prenylation / inhibitor / TRANSFERASE-TRANSFERASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationApoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / peptide pheromone maturation / protein farnesylation / protein geranylgeranyltransferase type I / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase ...Apoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / peptide pheromone maturation / protein farnesylation / protein geranylgeranyltransferase type I / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase / protein farnesyltransferase activity / protein farnesyltransferase complex / Rab geranylgeranyltransferase activity / protein geranylgeranylation / regulation of fibroblast proliferation / geranylgeranyl diphosphate synthase activity / positive regulation of skeletal muscle acetylcholine-gated channel clustering / acetyltransferase activator activity / microtubule associated complex / enzyme-linked receptor protein signaling pathway / regulation of microtubule-based movement / positive regulation of Rac protein signal transduction / alpha-tubulin binding / positive regulation of cell cycle / lipid metabolic process / wound healing / receptor tyrosine kinase binding / positive regulation of fibroblast proliferation / fibroblast proliferation / microtubule binding / molecular adaptor activity / cell population proliferation / negative regulation of cell population proliferation / positive regulation of cell population proliferation / negative regulation of apoptotic process / enzyme binding / zinc ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Guo, Z. / Stigter, E.A. / Bon, R.S. / Waldmann, H. / Blankenfeldt, W. / Goody, R.S. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2012 Journal: J.Med.Chem. / Year: 2012Title: Development of Selective, Potent RabGGTase Inhibitors Authors: Stigter, E.A. / Guo, Z. / Bon, R.S. / Wu, Y.W. / Choidas, A. / Wolf, A. / Menninger, S. / Waldmann, H. / Blankenfeldt, W. / Goody, R.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4gtr.cif.gz 4gtr.cif.gz | 324.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4gtr.ent.gz pdb4gtr.ent.gz | 263 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4gtr.json.gz 4gtr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gt/4gtr https://data.pdbj.org/pub/pdb/validation_reports/gt/4gtr ftp://data.pdbj.org/pub/pdb/validation_reports/gt/4gtr ftp://data.pdbj.org/pub/pdb/validation_reports/gt/4gtr | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4gtmC  4gtoC  4gtpC  4gtqC  4gtsC  4gttC  4gtvC  3eu5S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 44098.145 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: Q04631, protein farnesyltransferase, protein geranylgeranyltransferase type I |
|---|---|
| #2: Protein | Mass: 47749.340 Da / Num. of mol.: 1 / Fragment: C-terminal 10 residues deleted Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Non-polymers , 5 types, 411 molecules 

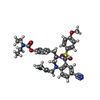
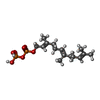





| #3: Chemical | | #4: Chemical | ChemComp-ZN / | #5: Chemical | ChemComp-7TR / | #6: Chemical | ChemComp-FPP / | #7: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.25 Å3/Da / Density % sol: 62.11 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 20% (w/v) PEG 4000, 0.2M MgCl2, 0.1M Tris, pH 7.5, VAPOR DIFFUSION, HANGING DROP, temperature 292K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 0.9786 Å / Beamline: X10SA / Wavelength: 0.9786 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: May 24, 2010 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9786 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→29.91 Å / Num. all: 60055 / Num. obs: 60023 / % possible obs: 99.9 % / Redundancy: 5.6 % / Biso Wilson estimate: 40.6 Å2 / Rsym value: 0.061 / Net I/σ(I): 18.8 |
| Reflection shell | Resolution: 2.2→2.3 Å / Redundancy: 5.5 % / Mean I/σ(I) obs: 4.5 / Rsym value: 0.387 / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3EU5 Resolution: 2.2→29.91 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.939 / SU B: 10.825 / SU ML: 0.123 / Cross valid method: THROUGHOUT / ESU R: 0.182 / ESU R Free: 0.16 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 42.553 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→29.91 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.2→2.257 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj





