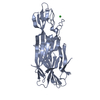
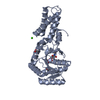
Entry Database : PDB / ID : 4eizTitle Structure of Nb113 bound to apoDHFR Dihydrofolate reductase Nb113 Camel antibody fragment Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Escherichia coli (E. coli)Lama glama (llama)Method / / / Resolution : 2.2 Å Authors Oyen, D. / Srinivasan, V. Journal : Biochim.Biophys.Acta / Year : 2013Title : Mechanistic analysis of allosteric and non-allosteric effects arising from nanobody binding to two epitopes of the dihyrofolate reductase of Escherichia coli.Authors : Oyen, D. / Wechselberger, R. / Srinivasan, V. / Steyaert, J. / Barlow, J.N. History Deposition Apr 6, 2012 Deposition site / Processing site Revision 1.0 Apr 24, 2013 Provider / Type Revision 1.1 Aug 14, 2013 Group Revision 1.2 Sep 4, 2013 Group Revision 1.3 Oct 30, 2013 Group Revision 1.4 Nov 20, 2024 Group / Database references / Structure summaryCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature Item / _database_2.pdbx_database_accession
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information

 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å
MOLECULAR REPLACEMENT / Resolution: 2.2 Å  Authors
Authors Citation
Citation Journal: Biochim.Biophys.Acta / Year: 2013
Journal: Biochim.Biophys.Acta / Year: 2013 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4eiz.cif.gz
4eiz.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4eiz.ent.gz
pdb4eiz.ent.gz PDB format
PDB format 4eiz.json.gz
4eiz.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ei/4eiz
https://data.pdbj.org/pub/pdb/validation_reports/ei/4eiz ftp://data.pdbj.org/pub/pdb/validation_reports/ei/4eiz
ftp://data.pdbj.org/pub/pdb/validation_reports/ei/4eiz Links
Links Assembly
Assembly


 Components
Components



 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID23-1 / Wavelength: 0.978 Å
/ Beamline: ID23-1 / Wavelength: 0.978 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.2→19.989 Å / SU ML: 0.24 / σ(F): 1.34 / Phase error: 25.16 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 2.2→19.989 Å / SU ML: 0.24 / σ(F): 1.34 / Phase error: 25.16 / Stereochemistry target values: ML Movie
Movie Controller
Controller




















 PDBj
PDBj


