[English] 日本語
 Yorodumi
Yorodumi- PDB-4c4s: Structure of beta-phosphoglucomutase in complex with an alpha- fl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4c4s | ||||||
|---|---|---|---|---|---|---|---|
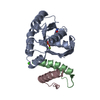
| Title | Structure of beta-phosphoglucomutase in complex with an alpha- fluorophosphonate analogue of beta-glucose-1-phosphate and magnesium trifluoride | ||||||
 Components Components | BETA-PHOSPHOGLUCOMUTASE | ||||||
 Keywords Keywords | ISOMERASE / PHOSPHORYL TRANSFER / TRANSITION STATE / METAL FLUORIDE / MUTASE | ||||||
| Function / homology |  Function and homology information Function and homology informationbeta-phosphoglucomutase / beta-phosphoglucomutase activity / carbohydrate metabolic process / magnesium ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  LACTOCOCCUS LACTIS (lactic acid bacteria) LACTOCOCCUS LACTIS (lactic acid bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | ||||||
 Authors Authors | Pellegrini, E. / Bowler, M.W. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2014 Journal: Proc.Natl.Acad.Sci.USA / Year: 2014Title: Alpha-Fluorophosphonates Reveal How a Phosphomutase Conserves Transition State Conformation Over Hexose Recognition in its Two-Step Reaction. Authors: Jin, Y. / Bhattasali, D. / Pellegrini, E. / Forget, S.M. / Baxter, N.J. / Cliff, M.J. / Bowler, M.W. / Jakeman, D.L. / Blackburn, G.M. / Waltho, J.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4c4s.cif.gz 4c4s.cif.gz | 64.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4c4s.ent.gz pdb4c4s.ent.gz | 45 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4c4s.json.gz 4c4s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c4/4c4s https://data.pdbj.org/pub/pdb/validation_reports/c4/4c4s ftp://data.pdbj.org/pub/pdb/validation_reports/c4/4c4s ftp://data.pdbj.org/pub/pdb/validation_reports/c4/4c4s | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2wf7C  4c4rC  4c4tC  2wf5S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 24239.594 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  LACTOCOCCUS LACTIS (lactic acid bacteria) LACTOCOCCUS LACTIS (lactic acid bacteria)Production host:  |
|---|---|
| #2: Sugar | ChemComp-GRX / ( |
| #3: Chemical | ChemComp-MGF / |
| #4: Chemical | ChemComp-MG / |
| #5: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.22 Å3/Da / Density % sol: 44.7 % Description: DATA WERE COLLECTED USING THE GROB ROBOT GONIOMETER |
|---|---|
| Crystal grow | pH: 7.2 Details: 27-32% PEG 4000, 50-75 MM MAGNESIUM ACETATE, pH 7.2 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-2 / Wavelength: 0.933 / Beamline: ID14-2 / Wavelength: 0.933 |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Oct 20, 2011 / Details: TOROIDAL MIRROR |
| Radiation | Monochromator: C001 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.933 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→52.2 Å / Num. obs: 33872 / % possible obs: 97.1 % / Observed criterion σ(I): 3 / Redundancy: 3.4 % / Biso Wilson estimate: 12.1 Å2 / Rmerge(I) obs: 0.04 / Net I/σ(I): 19.8 |
| Reflection shell | Resolution: 1.5→1.58 Å / Redundancy: 2.9 % / Rmerge(I) obs: 0.34 / Mean I/σ(I) obs: 3.2 / % possible all: 88.1 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2WF5 Resolution: 1.5→37.634 Å / SU ML: 0.18 / σ(F): 1.35 / Phase error: 18.22 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.98 Å / VDW probe radii: 1.2 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 39.199 Å2 / ksol: 0.394 e/Å3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 12.9 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→37.634 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj