[English] 日本語
 Yorodumi
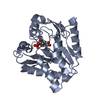
Yorodumi- PDB-4c1q: Crystal structure of the PRDM9 SET domain in complex with H3K4me2... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4c1q | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the PRDM9 SET domain in complex with H3K4me2 and AdoHcy. | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE/PEPTIDE / TRANSFERASE-PEPTIDE COMPLEX / HISTONE METHYLTRANSFERASE / SET DOMAIN H3K4ME3 | ||||||
| Function / homology |  Function and homology information Function and homology informationrecombination hotspot binding / positive regulation of reciprocal meiotic recombination / positive regulation of fertilization / male gamete generation / meiotic gene conversion / [histone H3]-lysine9 N-trimethyltransferase / [histone H4]-N-methyl-L-lysine20 N-methyltransferase / histone H4K20me methyltransferase activity / [histone H4]-lysine20 N-methyltransferase / histone H4K20 monomethyltransferase activity ...recombination hotspot binding / positive regulation of reciprocal meiotic recombination / positive regulation of fertilization / male gamete generation / meiotic gene conversion / [histone H3]-lysine9 N-trimethyltransferase / [histone H4]-N-methyl-L-lysine20 N-methyltransferase / histone H4K20me methyltransferase activity / [histone H4]-lysine20 N-methyltransferase / histone H4K20 monomethyltransferase activity / histone H3K9 trimethyltransferase activity / positive regulation of meiosis I / female gamete generation / [histone H3]-lysine36 N-trimethyltransferase / [histone H3]-lysine4 N-trimethyltransferase / PKMTs methylate histone lysines / histone H3K36 trimethyltransferase activity / double-strand break repair involved in meiotic recombination / homologous chromosome pairing at meiosis / histone H3K4 trimethyltransferase activity / histone H3K36 methyltransferase activity / meiosis I / histone H3K4 methyltransferase activity / Chromatin modifying enzymes / telomere organization / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / Assembly of the ORC complex at the origin of replication / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Transferases; Transferring one-carbon groups; Methyltransferases / Chromatin modifications during the maternal to zygotic transition (MZT) / HCMV Late Events / SIRT1 negatively regulates rRNA expression / epigenetic regulation of gene expression / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / HDACs deacetylate histones / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / HDMs demethylate histones / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / Meiotic recombination / Pre-NOTCH Transcription and Translation / RMTs methylate histone arginines / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Transcriptional regulation of granulopoiesis / HCMV Early Events / structural constituent of chromatin / nucleosome / nucleosome assembly / chromatin organization / HATs acetylate histones / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Factors involved in megakaryocyte development and platelet production / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / Senescence-Associated Secretory Phenotype (SASP) / Oxidative Stress Induced Senescence / gene expression / spermatogenesis / methylation / Estrogen-dependent gene expression / sequence-specific DNA binding / transcription cis-regulatory region binding / cadherin binding / Amyloid fiber formation / protein heterodimerization activity / negative regulation of apoptotic process / chromatin / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / protein-containing complex / DNA binding / extracellular exosome / extracellular region / zinc ion binding / nucleoplasm / nucleus / membrane Similarity search - Function | ||||||
| Biological species |   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Mathioudakis, N. / Cusack, S. / Kadlec, J. | ||||||
 Citation Citation |  Journal: Cell Rep. / Year: 2013 Journal: Cell Rep. / Year: 2013Title: Molecular Basis for the Regulation of the H3K4 Methyltransferase Activity of Prdm9. Authors: Wu, H. / Mathioudakis, N. / Diagouraga, B. / Dong, A. / Dombrovski, L. / Baudat, F. / Cusack, S. / De Massy, B. / Kadlec, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4c1q.cif.gz 4c1q.cif.gz | 87.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4c1q.ent.gz pdb4c1q.ent.gz | 63.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4c1q.json.gz 4c1q.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c1/4c1q https://data.pdbj.org/pub/pdb/validation_reports/c1/4c1q ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c1q ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c1q | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3rayS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: 1 / Refine code: 1
NCS oper: (Code: given Matrix: (0.998392, 0.034147, -0.045252), Vector: |
- Components
Components
-Protein / Protein/peptide , 2 types, 3 molecules ABC
| #1: Protein | Mass: 19899.029 Da / Num. of mol.: 2 / Fragment: SET DOMAIN, RESIDUES 198-368 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: Q96EQ9, histone-lysine N-methyltransferase #2: Protein/peptide | | Mass: 1253.474 Da / Num. of mol.: 1 / Fragment: N-TERMINUS, RESIDUES 2-11 / Source method: obtained synthetically / Details: DOUBLE METHYLATION ON LYSINE 4 / Source: (synth.)  HOMO SAPIENS (human) / References: UniProt: P68431 HOMO SAPIENS (human) / References: UniProt: P68431 |
|---|
-Non-polymers , 4 types, 65 molecules 






| #3: Chemical | ChemComp-SAH / | ||||
|---|---|---|---|---|---|
| #4: Chemical | | #5: Chemical | ChemComp-GOL / | #6: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.95 Å3/Da / Density % sol: 58 % / Description: NONE |
|---|---|
| Crystal grow | Temperature: 278 K / Method: vapor diffusion / pH: 5.5 Details: 0.2 M AMMONIUM SULFATE, 0.1 M BIS-TRIS PH 5.5 AND 25% W/V PEG3350 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.939 / Beamline: ID29 / Wavelength: 0.939 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Jul 2, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.939 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→100 Å / Num. obs: 21499 / % possible obs: 99.5 % / Observed criterion σ(I): 2.3 / Redundancy: 4.3 % / Rmerge(I) obs: 0.066 / Net I/σ(I): 13.7 |
| Reflection shell | Resolution: 2.3→2.38 Å / Redundancy: 4.4 % / Rmerge(I) obs: 0.644 / Mean I/σ(I) obs: 2.3 / % possible all: 99.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3RAY Resolution: 2.3→49.54 Å / Cor.coef. Fo:Fc: 0.953 / Cor.coef. Fo:Fc free: 0.935 / SU B: 8.231 / SU ML: 0.186 / Cross valid method: THROUGHOUT / ESU R: 0.274 / ESU R Free: 0.221 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 54.5 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→49.54 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj














