[English] 日本語
 Yorodumi
Yorodumi- PDB-4c1c: Crystal structure of the metallo-beta-lactamase BCII with D-captopril -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4c1c | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the metallo-beta-lactamase BCII with D-captopril | ||||||
 Components Components | BETA-LACTAMASE 2 | ||||||
 Keywords Keywords | HYDROLASE / MBL. METALLO-BETA-LACTAMASE / ANTIBIOTIC RESISTANCE | ||||||
| Function / homology |  Function and homology information Function and homology informationantibiotic catabolic process / beta-lactamase activity / beta-lactamase / periplasmic space / response to antibiotic / zinc ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.18 Å MOLECULAR REPLACEMENT / Resolution: 1.18 Å | ||||||
 Authors Authors | Zollman, D. / Brem, J. / McDonough, M.A. / van Berkel, S.S. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: Antimicrob. Agents Chemother. / Year: 2015 Journal: Antimicrob. Agents Chemother. / Year: 2015Title: Structural Basis of Metallo-beta-Lactamase Inhibition by Captopril Stereoisomers. Authors: Brem, J. / van Berkel, S.S. / Zollman, D. / Lee, S.Y. / Gileadi, O. / McHugh, P.J. / Walsh, T.R. / McDonough, M.A. / Schofield, C.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4c1c.cif.gz 4c1c.cif.gz | 117.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4c1c.ent.gz pdb4c1c.ent.gz | 89.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4c1c.json.gz 4c1c.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c1/4c1c https://data.pdbj.org/pub/pdb/validation_reports/c1/4c1c ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c1c ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c1c | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4bz3C  4c09C  4c1dC  4c1eC  4c1fC  4c1gC  4c1hC  1mqoS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 24995.533 Da / Num. of mol.: 1 / Fragment: RESIDUES 31-257 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 5 types, 272 molecules 

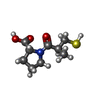






| #2: Chemical | | #3: Chemical | ChemComp-SO4 / | #4: Chemical | ChemComp-MCO / | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Nonpolymer details | ((S)-3-MERCAPTO-2-METHYLPROP |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 47 % / Description: NONE |
|---|---|
| Crystal grow | pH: 5.5 Details: 0.2 M AMMONIUM SULFATE, 0.1 M BIS TRIS, 25 % W/V PEG 3350, PH 5.5, 1 MM TCEP. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.9763 / Beamline: I03 / Wavelength: 0.9763 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 22, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9763 Å / Relative weight: 1 |
| Reflection | Resolution: 1.18→40.19 Å / Num. obs: 69596 / % possible obs: 95 % / Observed criterion σ(I): 0 / Redundancy: 4.5 % / Biso Wilson estimate: 13.6 Å2 / Rmerge(I) obs: 0.05 / Net I/σ(I): 11.5 |
| Reflection shell | Resolution: 1.18→1.21 Å / Redundancy: 4.4 % / Rmerge(I) obs: 0.6 / Mean I/σ(I) obs: 2.3 / % possible all: 90.4 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1MQO Resolution: 1.18→40.195 Å / SU ML: 0.11 / σ(F): 0.78 / Phase error: 12.4 / Stereochemistry target values: ML Details: CONFORMATION OF RAMACHANDRAN OUTLIERS 86 ASP, 87 SER AND 245 ASP VALIDATED BY CLEAR ELECTRON DENSITY. RAMACHANDRAN OUTLIER 117 ALA VALIDATED BY ELECTRON DENSITY AND IS CONSISTENT WITH ...Details: CONFORMATION OF RAMACHANDRAN OUTLIERS 86 ASP, 87 SER AND 245 ASP VALIDATED BY CLEAR ELECTRON DENSITY. RAMACHANDRAN OUTLIER 117 ALA VALIDATED BY ELECTRON DENSITY AND IS CONSISTENT WITH CONFORMATION IN PDB ENTRY 4C09. POSSIBLE OXIDISED CYS 198 AT VERY LOW OCCUPANCY LEFT UNMODELED DUE TO LOW OCCUPANCY. RESIDUES 41-44 AND 63-67 ARE DISORDERED WITH 64-66 UNMODELED.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 20.33 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.18→40.195 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





