[English] 日本語
 Yorodumi
Yorodumi- PDB-4c16: E-selectin lectin, EGF-like and two SCR domains complexed with gl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4c16 | ||||||
|---|---|---|---|---|---|---|---|
| Title | E-selectin lectin, EGF-like and two SCR domains complexed with glycomimetic antagonist | ||||||
 Components Components | E-SELECTIN | ||||||
 Keywords Keywords | CELL ADHESION / CELL-ADHESION MOLECULE / C-TYPE LECTIN / INFLAMMATION / LEUKOCYTE / GLYCOMIMETIC / ANTAGONIST / CATCH- BOND | ||||||
| Function / homology |  Function and homology information Function and homology informationactin filament-based process / positive regulation of leukocyte tethering or rolling / sialic acid binding / oligosaccharide binding / leukocyte migration involved in inflammatory response / leukocyte tethering or rolling / positive regulation of leukocyte migration / heterophilic cell-cell adhesion / leukocyte cell-cell adhesion / cortical cytoskeleton ...actin filament-based process / positive regulation of leukocyte tethering or rolling / sialic acid binding / oligosaccharide binding / leukocyte migration involved in inflammatory response / leukocyte tethering or rolling / positive regulation of leukocyte migration / heterophilic cell-cell adhesion / leukocyte cell-cell adhesion / cortical cytoskeleton / response to tumor necrosis factor / positive regulation of receptor internalization / phospholipase binding / response to cytokine / clathrin-coated pit / response to interleukin-1 / Cell surface interactions at the vascular wall / calcium-mediated signaling / caveola / transmembrane signaling receptor activity / regulation of inflammatory response / response to lipopolysaccharide / phospholipase C-activating G protein-coupled receptor signaling pathway / membrane raft / inflammatory response / external side of plasma membrane / perinuclear region of cytoplasm / extracellular space / metal ion binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.93 Å MOLECULAR REPLACEMENT / Resolution: 1.93 Å | ||||||
 Authors Authors | Preston, R.C. / Jakob, R.P. / Binder, F.P.C. / Sager, C.P. / Ernst, B. / Maier, T. | ||||||
 Citation Citation |  Journal: J.Mol.Cell.Biol. / Year: 2016 Journal: J.Mol.Cell.Biol. / Year: 2016Title: E-Selectin Ligand Complexes Adopt an Extended High-Affinity Conformation. Authors: Preston, R.C. / Jakob, R.P. / Binder, F.P. / Sager, C.P. / Ernst, B. / Maier, T. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: AUTHOR PROVIDED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4c16.cif.gz 4c16.cif.gz | 146.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4c16.ent.gz pdb4c16.ent.gz | 115.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4c16.json.gz 4c16.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c1/4c16 https://data.pdbj.org/pub/pdb/validation_reports/c1/4c16 ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c16 ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c16 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4csyC  1g1sS  1h04S  3govS  4c17 S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-1, -0.008697, -0.001289), Vector: |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 31305.760 Da / Num. of mol.: 2 Fragment: LECTIN DOMAIN, EGF-LIKE DOMAIN, SHORT CONSENSUS REPEAT DOMAIN 1, SHORT CONSENSUS REPEAT DOMAIN 2, RESDIUES 22-301 Source method: isolated from a genetically manipulated source Details: N-ACETYLGLUCOSAMINE RESIDUES ATTACHED TO ASN4, ASN124, ASN139, ASN158, ASN178, ASN182, AND ASN244 ON BOTH CHAINS. Source: (gene. exp.)  HOMO SAPIENS (human) / Tissue: CYTOKINE-INDUCED VASCULAR ENDOTHELIAL CELLS / Plasmid: PCDNA3.1 / Production host: HOMO SAPIENS (human) / Tissue: CYTOKINE-INDUCED VASCULAR ENDOTHELIAL CELLS / Plasmid: PCDNA3.1 / Production host:  |
|---|
-Sugars , 3 types, 18 molecules 
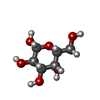
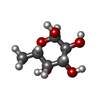


| #2: Sugar | ChemComp-NAG / #5: Sugar | #7: Sugar | |
|---|
-Non-polymers , 4 types, 531 molecules 






| #3: Chemical | | #4: Chemical | #6: Chemical | #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|---|
| Nonpolymer details | {(1R,2R, 3S)-2-[(ALPHA-L-FUCOPYRANOSYL)OXY]-3-METHYL-CYCLOHEX-1-YL} 3-O- [SODIUM (1S)-1-CARBOXY-2- ...{(1R,2R, 3S)-2-[(ALPHA-L-FUCOPYRANO |
| Sequence details | WITHOUT N-TERMINAL SECRETION SIGNAL (AA. 1-21). SEQUENCE OF MATURE PROTEIN STARTS WITH RESIDUE 1 ...WITHOUT N-TERMINAL SECRETION SIGNAL (AA. 1-21). SEQUENCE OF MATURE PROTEIN STARTS WITH RESIDUE 1 FOR COMPATIBIL |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.75 Å3/Da / Density % sol: 55.36 % / Description: NONE |
|---|---|
| Crystal grow | Details: PEG8000, HEPES, MOPS PH 6.2, CACL2, ANTAGONIST |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 0.99987 / Beamline: X06SA / Wavelength: 0.99987 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Feb 6, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.99987 Å / Relative weight: 1 |
| Reflection | Resolution: 1.93→57.43 Å / Num. obs: 50213 / % possible obs: 92.5 % / Observed criterion σ(I): 2 / Redundancy: 1.8 % / Biso Wilson estimate: 33.92 Å2 / Rmerge(I) obs: 0.04 / Net I/σ(I): 9.3 |
| Reflection shell | Resolution: 1.93→2.03 Å / Redundancy: 1.7 % / Rmerge(I) obs: 0.26 / Mean I/σ(I) obs: 2 / % possible all: 90.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRIES 1G1S,3GOV,1H04 Resolution: 1.93→15.81 Å / Cor.coef. Fo:Fc: 0.9325 / Cor.coef. Fo:Fc free: 0.9005 / SU R Cruickshank DPI: 0.166 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.172 / SU Rfree Blow DPI: 0.158 / SU Rfree Cruickshank DPI: 0.156
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 41.26 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.248 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.93→15.81 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.93→1.98 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj
















