[English] 日本語
 Yorodumi
Yorodumi- PDB-4bes: Crystal structure of the Legionella pneumophila FIC domain-contai... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4bes | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the Legionella pneumophila FIC domain-containing effector AnkX protein in complex with cytidine monophosphate and phosphocholine | ||||||
 Components Components | PHOSPHOCHOLINE TRANSFERASE ANKX | ||||||
 Keywords Keywords | TRANSFERASE / PHOSPHOCHOLINATION / TYPE IV SECRETION SYSTEM EFFECTOR | ||||||
| Function / homology |  Function and homology information Function and homology informationphosphocholine transferase activity / Transferases; Transferring phosphorus-containing groups; Phosphotransferases with an alcohol group as acceptor / regulation of GTPase activity / host cell cytoplasm / extracellular region Similarity search - Function | ||||||
| Biological species |  LEGIONELLA PNEUMOPHILA SUBSP. PNEUMOPHILA STR. PHILADELPHIA 1 (bacteria) LEGIONELLA PNEUMOPHILA SUBSP. PNEUMOPHILA STR. PHILADELPHIA 1 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.54 Å MOLECULAR REPLACEMENT / Resolution: 2.54 Å | ||||||
 Authors Authors | Campanacci, V. / Mukherjee, S. / Roy, C.R. / Cherfils, J. | ||||||
 Citation Citation |  Journal: Embo J. / Year: 2013 Journal: Embo J. / Year: 2013Title: Structure of the Legionella Effector Ankx Reveals the Mechanism of Phosphocholine Transfer by the Fic Domain. Authors: Campanacci, V. / Mukherjee, S. / Roy, C.R. / Cherfils, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4bes.cif.gz 4bes.cif.gz | 201.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4bes.ent.gz pdb4bes.ent.gz | 161.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4bes.json.gz 4bes.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4bes_validation.pdf.gz 4bes_validation.pdf.gz | 795.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4bes_full_validation.pdf.gz 4bes_full_validation.pdf.gz | 801.8 KB | Display | |
| Data in XML |  4bes_validation.xml.gz 4bes_validation.xml.gz | 18.8 KB | Display | |
| Data in CIF |  4bes_validation.cif.gz 4bes_validation.cif.gz | 25.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/be/4bes https://data.pdbj.org/pub/pdb/validation_reports/be/4bes ftp://data.pdbj.org/pub/pdb/validation_reports/be/4bes ftp://data.pdbj.org/pub/pdb/validation_reports/be/4bes | HTTPS FTP |
-Related structure data
| Related structure data |  4bepSC  4berC  4betC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 54889.660 Da / Num. of mol.: 1 / Fragment: FIC AND ANKYRIN REPEATS DOMAINS, RESIDUES 2-484 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  LEGIONELLA PNEUMOPHILA SUBSP. PNEUMOPHILA STR. PHILADELPHIA 1 (bacteria) LEGIONELLA PNEUMOPHILA SUBSP. PNEUMOPHILA STR. PHILADELPHIA 1 (bacteria)Production host:  References: UniProt: Q5ZXN6, Transferases; Transferring phosphorus-containing groups; Phosphotransferases with an alcohol group as acceptor | ||||||
|---|---|---|---|---|---|---|---|
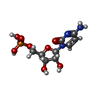
| #2: Chemical | ChemComp-C5P / | ||||||
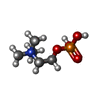
| #3: Chemical | ChemComp-PC / | ||||||
| #4: Chemical | | #5: Water | ChemComp-HOH / | Nonpolymer details | PHOSPHOCHOLINE (PC): PRODUCED AFTER ADDITION OF CDP-CHOLINE CYTIDINE-5'-MONOPHOSPHATE (C5P): ...PHOSPHOCHO | Sequence details | L247P (CLONING ARTEFACT) | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.43 Å3/Da / Density % sol: 49.45 % / Description: NONE |
|---|---|
| Crystal grow | pH: 6.5 Details: 0.2 M AMMONIUM SULFATE, 30% PEG 5000 MME, 0.1 M MES PH 6.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.9801 / Beamline: PROXIMA 1 / Wavelength: 0.9801 |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Mar 22, 2012 / Details: MIRRORS |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9801 Å / Relative weight: 1 |
| Reflection | Resolution: 2.54→43.1 Å / Num. obs: 19367 / % possible obs: 99.7 % / Redundancy: 5.8 % / Biso Wilson estimate: 55.92 Å2 / Rmerge(I) obs: 0.1 / Net I/σ(I): 13.42 |
| Reflection shell | Resolution: 2.54→2.68 Å / Redundancy: 5.7 % / Rmerge(I) obs: 0.75 / Mean I/σ(I) obs: 2.5 / % possible all: 98.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4BEP Resolution: 2.54→43.1 Å / Cor.coef. Fo:Fc: 0.9243 / Cor.coef. Fo:Fc free: 0.8889 / SU R Cruickshank DPI: 0.504 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.511 / SU Rfree Blow DPI: 0.289 / SU Rfree Cruickshank DPI: 0.292 Details: IDEAL-DIST CONTACT TERM CONTACT SETUP. ALL ATOMS HAVE CCP4 ATOM TYPE FROM LIBRARY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 55.86 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.328 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.54→43.1 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.54→2.68 Å / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj