[English] 日本語
 Yorodumi
Yorodumi- PDB-3tq3: Crystal structure of M-PMV dUTPase with a mixed population of sub... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3tq3 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of M-PMV dUTPase with a mixed population of substrate (dUPNPP) and post-inversion product (dUMP) in the active sites | ||||||
 Components Components | DEOXYURIDINE 5'-TRIPHOSPHATE NUCLEOTIDO HYDROLASE | ||||||
 Keywords Keywords | HYDROLASE / jelly roll | ||||||
| Function / homology |  Function and homology information Function and homology informationdUTP diphosphatase / dUTP diphosphatase activity / nucleotide metabolic process / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / viral nucleocapsid / structural constituent of virion / aspartic-type endopeptidase activity / viral translational frameshifting / proteolysis / DNA binding / zinc ion binding Similarity search - Function | ||||||
| Biological species |  Mason-Pfizer monkey virus Mason-Pfizer monkey virus | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / rigid body refinement / Resolution: 1.85 Å SYNCHROTRON / rigid body refinement / Resolution: 1.85 Å | ||||||
 Authors Authors | Barabas, O. / Nemeth, V. / Vertessy, B.G. | ||||||
 Citation Citation |  Journal: to be published Journal: to be publishedTitle: Structural Snapshots of Enzyme-Catalysed Phosphate Ester Hydrolysis Directly Visualize In-line Attack and Inversion Authors: Barabas, O. / Nemeth, V. / Bodor, A. / Perczel, A. / Rosta, E. / Kele, Z. / Zagyva, I. / Szabadka, Z. / Grolmusz, V.I. / Wilmanns, M. / Vertessy, B.G. #1: Journal: Acta Crystallogr.,Sect.F / Year: 2006 Title: Crystallization and preliminary X-ray studies of dUTPase from Mason-Pfizer monkey retrovirus. Authors: Barabas, O. / Nemeth, V. / Vertessy, B.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3tq3.cif.gz 3tq3.cif.gz | 66.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3tq3.ent.gz pdb3tq3.ent.gz | 47.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3tq3.json.gz 3tq3.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tq/3tq3 https://data.pdbj.org/pub/pdb/validation_reports/tq/3tq3 ftp://data.pdbj.org/pub/pdb/validation_reports/tq/3tq3 ftp://data.pdbj.org/pub/pdb/validation_reports/tq/3tq3 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3tp1C  3tpnC  3tpsC  3tpwC  3tpyC  3tq4C 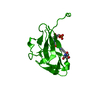 3tq5C  3trlC  2d4lS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 16155.373 Da / Num. of mol.: 1 / Fragment: dUTPase (catalytic) domain, UNP residues 608-759 / Mutation: N1K Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mason-Pfizer monkey virus / Gene: gag-pro / Plasmid: pET22B / Production host: Mason-Pfizer monkey virus / Gene: gag-pro / Plasmid: pET22B / Production host:  References: UniProt: O92810, UniProt: P07570*PLUS, dUTP diphosphatase |
|---|---|
| #2: Chemical | ChemComp-UMP / |
| #3: Chemical | ChemComp-DUP / |
| #4: Chemical | ChemComp-MG / |
| #5: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.1 Å3/Da / Density % sol: 41.55 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: PEG 8000, AMMONIUM CHLORIDE, TRIS, PH 8.5, vapor diffusion, hanging drop, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: X11 / Wavelength: 0.8124 Å / Beamline: X11 / Wavelength: 0.8124 Å |
| Detector | Type: MAR CCD 165 mm / Detector: CCD / Date: Nov 14, 2003 / Details: mirrors |
| Radiation | Monochromator: Si [111], horizontally focusing / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.8124 Å / Relative weight: 1 |
| Reflection | Resolution: 1.85→52.49 Å / Num. all: 11478 / Num. obs: 11478 / % possible obs: 99.8 % / Observed criterion σ(I): -3 / Redundancy: 11.2 % / Rsym value: 0.078 / Net I/σ(I): 20.2 |
| Reflection shell | Resolution: 1.85→1.96 Å / Redundancy: 11.2 % / Mean I/σ(I) obs: 5 / Num. unique all: 1821 / Rsym value: 0.539 / % possible all: 99.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: rigid body refinement Starting model: PDB entry 2D4L Resolution: 1.85→20 Å / Cor.coef. Fo:Fc: 0.97 / Cor.coef. Fo:Fc free: 0.961 / Occupancy max: 1 / Occupancy min: 0.3 / SU B: 5.208 / SU ML: 0.07 / Isotropic thermal model: TLS and isotropic individual / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.097 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 52.67 Å2 / Biso mean: 25.398 Å2 / Biso min: 13.38 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→20 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.85→1.898 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 2.4667 Å / Origin y: 58.4802 Å / Origin z: 30.3162 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller


















 PDBj
PDBj