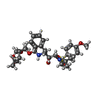
Entry Database : PDB / ID : 3ok9Title Crystal structure of wild-type HIV-1 protease with new oxatricyclic designed inhibitor GRL-0519A Protease Keywords / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Method / / / Resolution : 1.27 Å Authors Wang, Y.-F. / Agniswamy, J. / Weber, I.T. Journal : Chemmedchem / Year : 2010Title : Probing Multidrug-Resistance and Protein-Ligand Interactions with Oxatricyclic Designed Ligands in HIV-1 Protease Inhibitors.Authors : Ghosh, A.K. / Xu, C.X. / Rao, K.V. / Baldridge, A. / Agniswamy, J. / Wang, Y.F. / Weber, I.T. / Aoki, M. / Miguel, S.G. / Amano, M. / Mitsuya, H. History Deposition Aug 24, 2010 Deposition site / Processing site Revision 1.0 Sep 22, 2010 Provider / Type Revision 1.1 Jul 13, 2011 Group Revision 1.2 Aug 23, 2017 Group / Category / Item / _diffrn_detector.typeRevision 1.3 Sep 6, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 Human immunodeficiency virus 1
Human immunodeficiency virus 1 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON / Phaser / Resolution: 1.27 Å
SYNCHROTRON / Phaser / Resolution: 1.27 Å  Authors
Authors Citation
Citation Journal: Chemmedchem / Year: 2010
Journal: Chemmedchem / Year: 2010 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3ok9.cif.gz
3ok9.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3ok9.ent.gz
pdb3ok9.ent.gz PDB format
PDB format 3ok9.json.gz
3ok9.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ok/3ok9
https://data.pdbj.org/pub/pdb/validation_reports/ok/3ok9 ftp://data.pdbj.org/pub/pdb/validation_reports/ok/3ok9
ftp://data.pdbj.org/pub/pdb/validation_reports/ok/3ok9
 Links
Links Assembly
Assembly
 Components
Components
 Human immunodeficiency virus 1 / Gene: pol / Plasmid: pET11a / Production host:
Human immunodeficiency virus 1 / Gene: pol / Plasmid: pET11a / Production host: 








 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 22-ID / Wavelength: 0.8 / Wavelength: 0.8 Å
/ Beamline: 22-ID / Wavelength: 0.8 / Wavelength: 0.8 Å Processing
Processing Movie
Movie Controller
Controller




















 PDBj
PDBj






