[English] 日本語
 Yorodumi
Yorodumi- PDB-3gp1: MutM encountering an intrahelical 8-oxoguanine (oxoG) lesion in E... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3gp1 | ||||||
|---|---|---|---|---|---|---|---|
| Title | MutM encountering an intrahelical 8-oxoguanine (oxoG) lesion in EC3-V222P complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/DNA / DNA glycosylase / DNA repair / damage search / base extrusion / disulfide crosslinking / DNA damage / DNA-binding / Glycosidase / Hydrolase / Lyase / Metal-binding / Multifunctional enzyme / Zinc-finger / HYDROLASE-DNA COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationDNA-formamidopyrimidine glycosylase / 8-oxo-7,8-dihydroguanine DNA N-glycosylase activity / class I DNA-(apurinic or apyrimidinic site) endonuclease activity / DNA-(apurinic or apyrimidinic site) lyase / base-excision repair / double-stranded DNA binding / damaged DNA binding / zinc ion binding Similarity search - Function | ||||||
| Biological species |   Geobacillus stearothermophilus (bacteria) Geobacillus stearothermophilus (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.05 Å MOLECULAR REPLACEMENT / Resolution: 2.05 Å | ||||||
 Authors Authors | Spong, M.C. / Qi, Y. / Verdine, G.L. | ||||||
 Citation Citation |  Journal: Nature / Year: 2009 Journal: Nature / Year: 2009Title: Encounter and extrusion of an intrahelical lesion by a DNA repair enzyme Authors: Qi, Y. / Spong, M.C. / Nam, K. / Banerjee, A. / Jiralerspong, S. / Karplus, M. / Verdine, G.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3gp1.cif.gz 3gp1.cif.gz | 88 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3gp1.ent.gz pdb3gp1.ent.gz | 61.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3gp1.json.gz 3gp1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gp/3gp1 https://data.pdbj.org/pub/pdb/validation_reports/gp/3gp1 ftp://data.pdbj.org/pub/pdb/validation_reports/gp/3gp1 ftp://data.pdbj.org/pub/pdb/validation_reports/gp/3gp1 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3go8C  3gppC  3gpuC  3gpxC 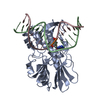 3gpyC  3gq3C  3gq4C  3gq5C  2f5oS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 30581.314 Da / Num. of mol.: 1 / Mutation: Q166C, V222P Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Geobacillus stearothermophilus (bacteria) Geobacillus stearothermophilus (bacteria)Gene: MutM / Plasmid: pET24B / Production host:  References: UniProt: P84131, DNA-(apurinic or apyrimidinic site) lyase |
|---|---|
| #2: DNA chain | Mass: 4948.217 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: synthetic DNA |
| #3: DNA chain | Mass: 4576.959 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: synthetic DNA |
| #4: Chemical | ChemComp-ZN / |
| #5: Water | ChemComp-HOH / |
| Sequence details | AUTHORS STATE THAT THE SEQUENCE IN THE UNP DATABASE IS NOT THE WILD-TYPE SEQUENCE, BUT HAS GLU 3 TO ...AUTHORS STATE THAT THE SEQUENCE IN THE UNP DATABASE IS NOT THE WILD-TYPE SEQUENCE, BUT HAS GLU 3 TO GLN 3 MUTATION THAT MAKES THE ENZYME INACTIVE. THE SEQUENCE WITH GLU AT POSITION 3 IS THE WILD-TYPE SEQUENCE. |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.88 Å3/Da / Density % sol: 57.34 % | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7 Details: PEG 8000, Sodium cacodylate, Glycerol, pH 7.0, VAPOR DIFFUSION, HANGING DROP, temperature 298K | ||||||||||||||||||||||||||||
| Components of the solutions |
|
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.979 Å / Beamline: 19-ID / Wavelength: 0.979 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Dec 15, 2006 Details: Rosenbaum-Rock vertical focusing mirror, with Pt, glass, Pd lanes. LN2 cooled first crystal, sagittal focusing 2nd crystal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Rosenbaum-Rock high-resolution double-crystal Si(111) Protocol: SINGLE WAVELENGTH / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.979 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.05→50 Å / Num. obs: 28412 / % possible obs: 99.1 % / Redundancy: 5.7 % / Rmerge(I) obs: 0.138 / Χ2: 1.073 / Net I/σ(I): 11.588 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 2F5O Resolution: 2.05→45.69 Å / Cor.coef. Fo:Fc: 0.947 / Cor.coef. Fo:Fc free: 0.933 / Occupancy max: 1 / Occupancy min: 0.5 / SU ML: 0.095 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.165 / ESU R Free: 0.149 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 112.65 Å2 / Biso mean: 32.956 Å2 / Biso min: 12.78 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.05→45.69 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.05→2.1 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj










































