Entry Database : PDB / ID : 3dzuTitle Intact PPAR gamma - RXR alpha Nuclear Receptor Complex on DNA bound with BVT.13, 9-cis Retinoic Acid and NCOA2 Peptide DNA (5'-D(*DCP*DAP*DAP*DAP*DCP*DTP*DAP*DGP*DGP*DTP*DCP*DAP*DAP*DAP*DGP*DGP*DTP*DCP*DAP*DG)-3')DNA (5'-D(*DCP*DTP*DGP*DAP*DCP*DCP*DTP*DTP*DTP*DGP*DAP*DCP*DCP*DTP*DAP*DGP*DTP*DTP*DTP*DG)-3')NCOA2 Peptide Peroxisome proliferator-activated receptor gamma Retinoic acid receptor RXR-alpha Keywords / / / / / / / / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 3.2 Å Authors Chandra, V. / Huang, P. / Hamuro, Y. / Raghuram, S. / Wang, Y. / Burris, T.P. / Rastinejad, F. Journal : Nature / Year : 2008Title : Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA.Authors : Chandra, V. / Huang, P. / Hamuro, Y. / Raghuram, S. / Wang, Y. / Burris, T.P. / Rastinejad, F. History Deposition Jul 30, 2008 Deposition site / Processing site Revision 1.0 Oct 28, 2008 Provider / Type Revision 1.1 Jul 13, 2011 Group Revision 1.2 May 16, 2012 Group Revision 1.3 Nov 16, 2016 Group Revision 1.4 Oct 25, 2017 Group / Category Revision 1.5 Feb 21, 2024 Group / Database references / Derived calculationsCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.2 Å
MOLECULAR REPLACEMENT / Resolution: 3.2 Å  Authors
Authors Citation
Citation Journal: Nature / Year: 2008
Journal: Nature / Year: 2008 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3dzu.cif.gz
3dzu.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3dzu.ent.gz
pdb3dzu.ent.gz PDB format
PDB format 3dzu.json.gz
3dzu.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/dz/3dzu
https://data.pdbj.org/pub/pdb/validation_reports/dz/3dzu ftp://data.pdbj.org/pub/pdb/validation_reports/dz/3dzu
ftp://data.pdbj.org/pub/pdb/validation_reports/dz/3dzu Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: RXRA, NR2B1 / Plasmid: pET-46 Ek/LIC / Production host:
Homo sapiens (human) / Gene: RXRA, NR2B1 / Plasmid: pET-46 Ek/LIC / Production host: 
 Homo sapiens (human) / Gene: PPARG, NR1C3 / Plasmid: pET-46 Ek/LIC / Production host:
Homo sapiens (human) / Gene: PPARG, NR1C3 / Plasmid: pET-46 Ek/LIC / Production host: 


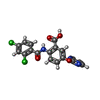


 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 22-ID / Wavelength: 1 Å
/ Beamline: 22-ID / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 3.2→38.93 Å / Occupancy max: 1 / Occupancy min: 1 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
MOLECULAR REPLACEMENT / Resolution: 3.2→38.93 Å / Occupancy max: 1 / Occupancy min: 1 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber Movie
Movie Controller
Controller














 PDBj
PDBj













































