[English] 日本語
 Yorodumi
Yorodumi- PDB-2ybb: Fitted model for bovine mitochondrial supercomplex I1III2IV1 by s... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2ybb | ||||||
|---|---|---|---|---|---|---|---|
| Title | Fitted model for bovine mitochondrial supercomplex I1III2IV1 by single particle cryo-EM (EMD-1876) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | OXIDOREDUCTASE / SUPERCOMPLEX B / MITOCHONDRIA / RESPIRATORY CHAIN / AMPHIPOL A8-35 / RANDOM CONICAL TILT | ||||||
| Function / homology |  Function and homology information Function and homology informationRelease of apoptotic factors from the mitochondria / Formation of apoptosome / Activation of caspases through apoptosome-mediated cleavage / Pyroptosis / Regulation of the apoptosome activity / Transcriptional activation of mitochondrial biogenesis / Detoxification of Reactive Oxygen Species / Complex III assembly / Complex IV assembly / TP53 Regulates Metabolic Genes ...Release of apoptotic factors from the mitochondria / Formation of apoptosome / Activation of caspases through apoptosome-mediated cleavage / Pyroptosis / Regulation of the apoptosome activity / Transcriptional activation of mitochondrial biogenesis / Detoxification of Reactive Oxygen Species / Complex III assembly / Complex IV assembly / TP53 Regulates Metabolic Genes / respiratory chain complex IV assembly / Cytoprotection by HMOX1 / subthalamus development / pons development / Translocases; Catalysing the translocation of protons; Linked to oxidoreductase reactions / NADH dehydrogenase (quinone) (non-electrogenic) activity / mitochondrial respirasome assembly / cerebellar Purkinje cell layer development / Respiratory electron transport / pyramidal neuron development / thalamus development / respiratory chain complex IV / molybdopterin cofactor binding / respiratory chain complex / cytochrome-c oxidase / respiratory chain complex III / oxidative phosphorylation / quinol-cytochrome-c reductase / mitochondrial electron transport, cytochrome c to oxygen / quinol-cytochrome-c reductase activity / cytochrome-c oxidase activity / mitochondrial electron transport, ubiquinol to cytochrome c / iron-sulfur cluster assembly / Mitochondrial protein degradation / hypothalamus development / midbrain development / ubiquinone binding / electron transport coupled proton transport / NADH dehydrogenase activity / respiratory chain complex I / NADH dehydrogenase (ubiquinone) activity / quinone binding / enzyme regulator activity / ATP synthesis coupled electron transport / ferric iron binding / aerobic respiration / central nervous system development / respiratory electron transport chain / hippocampus development / metalloendopeptidase activity / mitochondrial intermembrane space / 2 iron, 2 sulfur cluster binding / mitochondrial membrane / NAD binding / FMN binding / 4 iron, 4 sulfur cluster binding / electron transfer activity / oxidoreductase activity / mitochondrial inner membrane / iron ion binding / copper ion binding / apoptotic process / heme binding / mitochondrion / proteolysis / metal ion binding / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |   THERMUS THERMOPHILUS (bacteria) THERMUS THERMOPHILUS (bacteria)  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 19 Å | ||||||
 Authors Authors | Althoff, T. / Mills, D.J. / Popot, J.-L. / Kuehlbrandt, W. | ||||||
 Citation Citation |  Journal: EMBO J / Year: 2011 Journal: EMBO J / Year: 2011Title: Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. Authors: Thorsten Althoff / Deryck J Mills / Jean-Luc Popot / Werner Kühlbrandt /  Abstract: The respiratory chain in the inner mitochondrial membrane contains three large multi-enzyme complexes that together establish the proton gradient for ATP synthesis, and assemble into a supercomplex. ...The respiratory chain in the inner mitochondrial membrane contains three large multi-enzyme complexes that together establish the proton gradient for ATP synthesis, and assemble into a supercomplex. A 19-Å 3D map of the 1.7-MDa amphipol-solubilized supercomplex I(1)III(2)IV(1) from bovine heart obtained by single-particle electron cryo-microscopy reveals an amphipol belt replacing the membrane lipid bilayer. A precise fit of the X-ray structures of complex I, the complex III dimer, and monomeric complex IV indicates distances of 13 nm between the ubiquinol-binding sites of complexes I and III, and of 10-11 nm between the cytochrome c binding sites of complexes III and IV. The arrangement of respiratory chain complexes suggests two possible pathways for efficient electron transfer through the supercomplex, of which the shorter branch through the complex III monomer proximal to complex I may be preferred. #1:  Journal: Ph D Thesis / Year: 2011 Journal: Ph D Thesis / Year: 2011Title: Strukturelle Untersuchungen Am Superkomplex I1III2Iv1 Der Atmungskette Mittels Kryoelektronenmikroskopie Authors: Althoff, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2ybb.cif.gz 2ybb.cif.gz | 1.8 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2ybb.ent.gz pdb2ybb.ent.gz | 1.4 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2ybb.json.gz 2ybb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yb/2ybb https://data.pdbj.org/pub/pdb/validation_reports/yb/2ybb ftp://data.pdbj.org/pub/pdb/validation_reports/yb/2ybb ftp://data.pdbj.org/pub/pdb/validation_reports/yb/2ybb | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1876MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-NADH-QUINONE OXIDOREDUCTASE SUBUNIT ... , 10 types, 10 molecules 12345678op
| #1: Protein | Mass: 48693.715 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO1 (51KDA) / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56222, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56222, NADH dehydrogenase (quinone) |
|---|---|
| #2: Protein | Mass: 20309.162 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO2 (24KDA) / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56221, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56221, NADH dehydrogenase (quinone) |
| #3: Protein | Mass: 86656.203 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO3 (75KDA) / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56223, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56223, NADH dehydrogenase (quinone) |
| #4: Protein | Mass: 46428.027 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO4 (49KDA) / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56220, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56220, NADH dehydrogenase (quinone) |
| #5: Protein | Mass: 23893.254 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO5 (30KDA) / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56219, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56219, NADH dehydrogenase (quinone) |
| #6: Protein | Mass: 20262.564 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO6 (PSST) / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56218, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56218, NADH dehydrogenase (quinone) |
| #7: Protein | Mass: 14812.074 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO15 / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q5SKZ7, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q5SKZ7, NADH dehydrogenase (quinone) |
| #8: Protein | Mass: 20106.309 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: NQO9 (TYKY) / Source: (natural)   THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56224, NADH dehydrogenase (quinone) THERMUS THERMOPHILUS (bacteria) / Strain: HB8 / References: UniProt: Q56224, NADH dehydrogenase (quinone) |
| #36: Protein | Mass: 32187.574 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: SUBUNIT NUON (CHAIN2) / Source: (natural)  |
| #37: Protein | Mass: 23932.375 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: SUBUNITS NUOA, J AND K (CHAIN3,6 AND 4L) / Source: (natural)  |
-CYTOCHROME B-C1 COMPLEX SUBUNIT ... , 9 types, 18 molecules AaBbEeFfGgHhIiJjKk
| #9: Protein | Mass: 49266.254 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #10: Protein | Mass: 46575.469 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #13: Protein | Mass: 21640.580 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #14: Protein | Mass: 13371.190 Da / Num. of mol.: 2 / Mutation: YES / Source method: isolated from a natural source / Source: (natural)  #15: Protein | Mass: 9606.027 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #16: Protein | Mass: 9189.116 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #17: Protein | Mass: 6677.736 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #18: Protein | Mass: 7209.311 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #19: Protein | Mass: 6370.390 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Protein , 4 types, 6 molecules CcDdRY
| #11: Protein | Mass: 42620.340 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #12: Protein | Mass: 27323.277 Da / Num. of mol.: 2 / Fragment: RESIDUES 85-325 / Source method: isolated from a natural source / Source: (natural)  #26: Protein | | Mass: 9452.687 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  #33: Protein | | Mass: 11595.392 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-CYTOCHROME C OXIDASE SUBUNIT ... , 12 types, 12 molecules LMNOPQSTUVWX
| #20: Protein | Mass: 57065.844 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #21: Protein | Mass: 26040.393 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #22: Protein | Mass: 29957.627 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #23: Protein | Mass: 17179.646 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #24: Protein | Mass: 12453.081 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #25: Protein | Mass: 10684.038 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #27: Protein | Mass: 10039.244 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #28: Protein | Mass: 8494.982 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #29: Protein | Mass: 6682.726 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #30: Protein | Mass: 6365.217 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #31: Protein/peptide | Mass: 5449.396 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #32: Protein/peptide | Mass: 4967.756 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-NADH\: UBIQUINONE OXIDOREDUCTASE, MEMBRANE SUBUNIT ... , 2 types, 2 molecules mn
| #34: Protein | Mass: 40357.742 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: SUBUNIT NUOL (CHAIN5) / Source: (natural)  |
|---|---|
| #35: Protein | Mass: 33293.949 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: SUBUNIT NUOM (CHAIN4) / Source: (natural)  |
-Non-polymers , 15 types, 1640 molecules 






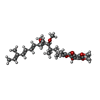





















| #38: Chemical | ChemComp-SF4 / #39: Chemical | ChemComp-FMN / | #40: Chemical | ChemComp-NAI / | #41: Chemical | ChemComp-MG / #42: Chemical | ChemComp-FES / #43: Chemical | ChemComp-CA / | #44: Chemical | ChemComp-HEM / #45: Chemical | #46: Chemical | #47: Chemical | #48: Chemical | ChemComp-CDL / #49: Chemical | #50: Chemical | #51: Chemical | ChemComp-ZN / | #52: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|---|
| Sequence details | DUE TO THE AMBIGUITY ABOUT THE REGISTER OF THE SEQUENCE, THESE CHAINS WERE BUILT WITH UNK RESIDUES ...DUE TO THE AMBIGUITY ABOUT THE REGISTER OF THE SEQUENCE, THESE CHAINS WERE BUILT WITH UNK RESIDUES ONLY. THE UNIPROT REFERENCES |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: RESPIRATORY SUPERCOMPLEX B (I1III2IV1) COMPOSED OF COMPLEX I, DIMERIC COMPLEX III AND COMPLEX IV FROM BOVINE HEART MITOCHONDRIA Type: COMPLEX Details: THE SUPERCOMPLEXES WERE KEPT SOLUBLE BY AMPHIPOLS A8-35 |
|---|---|
| Buffer solution | Name: 10 MM KCL, 15 MM HEPES, RESIDUAL TREHALOSE / pH: 7.7 / Details: 10 MM KCL, 15 MM HEPES, RESIDUAL TREHALOSE |
| Specimen | Conc.: 0.3 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Details: CARBON |
| Vitrification | Instrument: HOMEMADE PLUNGER / Cryogen name: ETHANE Details: VITRIFICATION 1 -- CRYOGEN- ETHANE, HUMIDITY- 70, TEMPERATURE- 95, INSTRUMENT- CUSTOM-MADE HAND PLUNGER, METHOD- ONE- SIDED BLOTTING FOR 26 - 30 SECONDS BEFORE PLUNGING, DETAILS- SAMPLE WAS ...Details: VITRIFICATION 1 -- CRYOGEN- ETHANE, HUMIDITY- 70, TEMPERATURE- 95, INSTRUMENT- CUSTOM-MADE HAND PLUNGER, METHOD- ONE- SIDED BLOTTING FOR 26 - 30 SECONDS BEFORE PLUNGING, DETAILS- SAMPLE WAS ADSORBED TO CARBON FILM FOR 30 SECONDS BEFORE BLOTTING |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TECNAI F30 Details: LOW DOSE CONDITIONS. FOR RANDOM CONICAL TILT EACH AREA WAS IMAGED TWICE WITH THE FIRST IMAGE TAKEN AT A TILT ANGLE OF -45 OR -50 DEGREE |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 59000 X / Calibrated magnification: 58829 X / Nominal defocus max: 6000 nm / Nominal defocus min: 500 nm / Cs: 2 mm |
| Specimen holder | Temperature: 78 K / Tilt angle max: 0 ° / Tilt angle min: -50 ° |
| Image recording | Electron dose: 25 e/Å2 / Film or detector model: KODAK SO-163 FILM |
| Image scans | Num. digital images: 154 |
| Radiation wavelength | Relative weight: 1 |
- Processing
Processing
| EM software |
| |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Details: PHASE-FLIPPPING ON EACH PARTICLE | |||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | |||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Method: RANDOM CONICAL TILT AND PROJECTION MATCHING WITH DIRECT FOURIER BACKPROJECTION AND SIRT Resolution: 19 Å / Num. of particles: 10684 / Nominal pixel size: 4.76 Å / Actual pixel size: 4.76 Å Details: FINAL MAP WAS CALCULATED WITH SIRT FROM COMBINED TILTED AND UNTILTED DATASETS WITH 10684 PARTICLES EACH AND OMITTING 30 PER CENT OF IMAGES WITH LOWEST CROSS CORRELATION. THIS ENTRY IS BASED ...Details: FINAL MAP WAS CALCULATED WITH SIRT FROM COMBINED TILTED AND UNTILTED DATASETS WITH 10684 PARTICLES EACH AND OMITTING 30 PER CENT OF IMAGES WITH LOWEST CROSS CORRELATION. THIS ENTRY IS BASED ON PDBS 3IAM,3M9C,1PP9,1BGY,1OCC AND 2B4Z WHICH WERE FITTED MANUALLY INTO THE EM DENSITY MAP OF BOVINE SUPERCOMPLEX I1III2IV1. THE POSITION OF 2B4Z WAS INFERRED FROM PDB 3CX5. THE CHAIN NAMES ARE CASE SENSITIVE. CHAINS A-K AND a-k DENOTE IDENTICAL CHAINS WITHIN A DIMER. CHAINS L-X AND M-P BELONG TO DIFFERENT PROTEINS. SUBMISSION BASED ON EXPERIMENTAL DATA FROM EMDB EMD-1876. Symmetry type: POINT | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL / Details: METHOD--RIGID BODY REFINEMENT PROTOCOL--X-RAY | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Highest resolution: 19 Å | |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 19 Å
|
 Movie
Movie Controller
Controller








 PDBj
PDBj








































