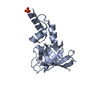
Entry Database : PDB / ID : 2xxnTitle Structure of the vIRF4-HAUSP TRAF domain complex K10 UBIQUITIN CARBOXYL-TERMINAL HYDROLASE 7 Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species HOMO SAPIENS (human)Method / / / Resolution : 1.6 Å Authors Choi, W.C. / Hwang, J. / Kim, M.H. Journal : Nat.Struct.Mol.Biol. / Year : 2011Title : Bilateral Inhibition of Hausp Deubiquitinase by a Viral Interferon Regulatory Factor ProteinAuthors : Lee, H.R. / Choi, W.C. / Lee, S. / Hwang, J. / Hwang, E. / Guchhait, K. / Haas, J. / Toth, Z. / Jeon, Y.H. / Oh, T.K. / Kim, M.H. / Jung, J.U. History Deposition Nov 11, 2010 Deposition site / Processing site Revision 1.0 Nov 9, 2011 Provider / Type Revision 1.1 Dec 14, 2011 Group Revision 1.2 Mar 28, 2012 Group Revision 1.3 Dec 20, 2023 Group Data collection / Database references ... Data collection / Database references / Other / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_database_status / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_sf
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information HOMO SAPIENS (human)
HOMO SAPIENS (human)
 HUMAN HERPESVIRUS 8
HUMAN HERPESVIRUS 8 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.6 Å
MOLECULAR REPLACEMENT / Resolution: 1.6 Å  Authors
Authors Citation
Citation Journal: Nat.Struct.Mol.Biol. / Year: 2011
Journal: Nat.Struct.Mol.Biol. / Year: 2011 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2xxn.cif.gz
2xxn.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2xxn.ent.gz
pdb2xxn.ent.gz PDB format
PDB format 2xxn.json.gz
2xxn.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 2xxn_validation.pdf.gz
2xxn_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 2xxn_full_validation.pdf.gz
2xxn_full_validation.pdf.gz 2xxn_validation.xml.gz
2xxn_validation.xml.gz 2xxn_validation.cif.gz
2xxn_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/xx/2xxn
https://data.pdbj.org/pub/pdb/validation_reports/xx/2xxn ftp://data.pdbj.org/pub/pdb/validation_reports/xx/2xxn
ftp://data.pdbj.org/pub/pdb/validation_reports/xx/2xxn
 Links
Links Assembly
Assembly
 Components
Components HOMO SAPIENS (human) / Production host:
HOMO SAPIENS (human) / Production host: 

 HUMAN HERPESVIRUS 8 / Production host:
HUMAN HERPESVIRUS 8 / Production host: 
 X-RAY DIFFRACTION
X-RAY DIFFRACTION Sample preparation
Sample preparation SYNCHROTRON / Site: PAL/PLS
SYNCHROTRON / Site: PAL/PLS  / Beamline: 4A / Wavelength: 0.9999
/ Beamline: 4A / Wavelength: 0.9999  Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller




















 PDBj
PDBj



