[English] 日本語
 Yorodumi
Yorodumi- PDB-2w6t: Structures of P. aeruginosa FpvA bound to heterologous pyoverdine... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2w6t | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structures of P. aeruginosa FpvA bound to heterologous pyoverdines: FpvA-Pvd(DSM50106)-Fe complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | MEMBRANE PROTEIN / RECEPTOR / TONB BOX / TRANSPORT / SIDEROPHORE / CELL MEMBRANE / ION TRANSPORT / IRON TRANSPORT / OUTER MEMBRANE / TONB-DEPENDENT TRANSPORTER | ||||||
| Function / homology |  Function and homology information Function and homology informationpyoverdine biosynthetic process / siderophore-iron import into cell / siderophore uptake transmembrane transporter activity / cell outer membrane / signaling receptor activity / membrane Similarity search - Function | ||||||
| Biological species |   PSEUDOMONAS FLUORESCENS (bacteria) PSEUDOMONAS FLUORESCENS (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.9 Å MOLECULAR REPLACEMENT / Resolution: 2.9 Å | ||||||
 Authors Authors | Greenwald, J. / Nader, M. / Celia, H. / Gruffaz, C. / Meyer, J.-M. / Schalk, I.J. / Pattus, F. | ||||||
 Citation Citation |  Journal: Mol.Microbiol. / Year: 2009 Journal: Mol.Microbiol. / Year: 2009Title: Fpva Bound to Non-Cognate Pyoverdines: Molecular Basis of Siderophore Recognition by an Iron Transporter. Authors: Greenwald, J. / Nader, M. / Celia, H. / Gruffaz, C. / Geoffroy, V. / Meyer, J.M. / Schalk, I.J. / Pattus, F. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AE" IN EACH CHAIN ON SHEET RECORDS BELOW ... SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AE" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 22-STRANDED BARREL THIS IS REPRESENTED BY A 23-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE SHEET RECORDS BELOW, TWO SHEETS ARE DEFINED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2w6t.cif.gz 2w6t.cif.gz | 314.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2w6t.ent.gz pdb2w6t.ent.gz | 252.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2w6t.json.gz 2w6t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2w6t_validation.pdf.gz 2w6t_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2w6t_full_validation.pdf.gz 2w6t_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  2w6t_validation.xml.gz 2w6t_validation.xml.gz | 56 KB | Display | |
| Data in CIF |  2w6t_validation.cif.gz 2w6t_validation.cif.gz | 76 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/w6/2w6t https://data.pdbj.org/pub/pdb/validation_reports/w6/2w6t ftp://data.pdbj.org/pub/pdb/validation_reports/w6/2w6t ftp://data.pdbj.org/pub/pdb/validation_reports/w6/2w6t | HTTPS FTP |
-Related structure data
| Related structure data |  2w16C  2w6uC  2w75C  2w76C  2w77C  2w78C  2o5pS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 3 molecules ABC
-Non-polymers , 4 types, 14 molecules 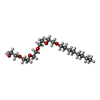






| #3: Chemical | | #4: Chemical | ChemComp-PO4 / #5: Chemical | ChemComp-PVE / ( |   Type: Polypeptide / Class: Metal transport / Mass: 376.341 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C17H18N3O7 Type: Polypeptide / Class: Metal transport / Mass: 376.341 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C17H18N3O7Details: IN IRON-DEFICIENT CONDITIONS, PSEUDOMONAS AERUGINOSA SECRETES A MAJOR FLUORESCENT SIDEROPHORE NAMED PYOVERDIN (PVD), WHICH AFTER CHELATING IRON(III) IS TRANSPORTED BACK INTO THE CELL VIA ITS ...Details: IN IRON-DEFICIENT CONDITIONS, PSEUDOMONAS AERUGINOSA SECRETES A MAJOR FLUORESCENT SIDEROPHORE NAMED PYOVERDIN (PVD), WHICH AFTER CHELATING IRON(III) IS TRANSPORTED BACK INTO THE CELL VIA ITS OUTER MEMBRANE RECEPTOR FPVA. FPVA IS A TONB-DEPENDENT TRANSPORT PROTEIN AND HAS THE ABILITY TO BIND PVD IN ITS APO- OR IRON-LOADED FORM. References: Synthetic PYOVERDINE Fe Complex #6: Chemical | ChemComp-FE / |   Type: Polypeptide / Class: Metal transport / Mass: 55.845 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: Fe Type: Polypeptide / Class: Metal transport / Mass: 55.845 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: FeDetails: IN IRON-DEFICIENT CONDITIONS, PSEUDOMONAS AERUGINOSA SECRETES A MAJOR FLUORESCENT SIDEROPHORE NAMED PYOVERDIN (PVD), WHICH AFTER CHELATING IRON(III) IS TRANSPORTED BACK INTO THE CELL VIA ITS ...Details: IN IRON-DEFICIENT CONDITIONS, PSEUDOMONAS AERUGINOSA SECRETES A MAJOR FLUORESCENT SIDEROPHORE NAMED PYOVERDIN (PVD), WHICH AFTER CHELATING IRON(III) IS TRANSPORTED BACK INTO THE CELL VIA ITS OUTER MEMBRANE RECEPTOR FPVA. FPVA IS A TONB-DEPENDENT TRANSPORT PROTEIN AND HAS THE ABILITY TO BIND PVD IN ITS APO- OR IRON-LOADED FORM. References: Synthetic PYOVERDINE Fe Complex |
|---|
-Details
| Compound details | PYOVERDINES ARE A GROUP OF STRUCTURALLY RELATED SIDEROPHORES PRODUCED BY FLUORESCENT PSEUDOMONAS ...PYOVERDINE |
|---|---|
| Nonpolymer details | C8E5 (N8E): DETERGENT |
| Sequence details | SIGNAL PEPTIDE (RESIDUES 1-43) IS NOT IN THE CRYSTALLIZ |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.85 Å3/Da / Density % sol: 67.82 % / Description: NONE |
|---|---|
| Crystal grow | Details: 1.3M NAH2PO4, 0.1M MES, PH 6.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: BW7B / Wavelength: 0.8423 / Beamline: BW7B / Wavelength: 0.8423 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.8423 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→107 Å / Num. obs: 54303 / % possible obs: 93.7 % / Redundancy: 2.4 % / Rmerge(I) obs: 0.11 / Net I/σ(I): 7.6 |
| Reflection shell | Resolution: 2.9→3.06 Å / Redundancy: 2.4 % / Rmerge(I) obs: 0.38 / Mean I/σ(I) obs: 2.3 / % possible all: 94.2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2O5P Resolution: 2.9→106 Å / Cor.coef. Fo:Fc: 0.915 / Cor.coef. Fo:Fc free: 0.853 / SU B: 30.04 / SU ML: 0.266 / TLS residual ADP flag: UNVERIFIED / Cross valid method: THROUGHOUT / ESU R: 1.478 / ESU R Free: 0.389 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 16.71 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.9→106 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj