[English] 日本語
 Yorodumi
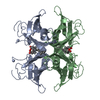
Yorodumi- PDB-2try: TERTIARY STRUCTURES OF THREE AMYLOIDOGENIC TRANSTHYRETIN VARIANTS... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2try | ||||||
|---|---|---|---|---|---|---|---|
| Title | TERTIARY STRUCTURES OF THREE AMYLOIDOGENIC TRANSTHYRETIN VARIANTS AND IMPLICATIONS FOR AMYLOID FIBRIL FORMATION | ||||||
 Components Components | TRANSTHYRETIN | ||||||
 Keywords Keywords | TRANSPORT / ALBUMIN / RETINOL-BINDING / VITAMIN A / AMYLOID / THYROID HORMONE / LIVER / PLASMA / CEREBROSPINAL FLUID / POLYNEUROPATHY / DISEASE MUTATION / THYROXINE / PREALBUMIN | ||||||
| Function / homology |  Function and homology information Function and homology informationDefective visual phototransduction due to STRA6 loss of function / negative regulation of glomerular filtration / The canonical retinoid cycle in rods (twilight vision) / purine nucleobase metabolic process / hormone binding / Non-integrin membrane-ECM interactions / molecular sequestering activity / phototransduction, visible light / retinoid metabolic process / Retinoid metabolism and transport ...Defective visual phototransduction due to STRA6 loss of function / negative regulation of glomerular filtration / The canonical retinoid cycle in rods (twilight vision) / purine nucleobase metabolic process / hormone binding / Non-integrin membrane-ECM interactions / molecular sequestering activity / phototransduction, visible light / retinoid metabolic process / Retinoid metabolism and transport / hormone activity / azurophil granule lumen / Amyloid fiber formation / Neutrophil degranulation / protein-containing complex binding / protein-containing complex / extracellular space / extracellular exosome / extracellular region / identical protein binding Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Schormann, N. / Murrell, J.R. / Benson, M.D. | ||||||
 Citation Citation |  Journal: Amyloid / Year: 1998 Journal: Amyloid / Year: 1998Title: Tertiary structures of amyloidogenic and non-amyloidogenic transthyretin variants: new model for amyloid fibril formation. Authors: Schormann, N. / Murrell, J.R. / Benson, M.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2try.cif.gz 2try.cif.gz | 59.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2try.ent.gz pdb2try.ent.gz | 44.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2try.json.gz 2try.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tr/2try https://data.pdbj.org/pub/pdb/validation_reports/tr/2try ftp://data.pdbj.org/pub/pdb/validation_reports/tr/2try ftp://data.pdbj.org/pub/pdb/validation_reports/tr/2try | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1b0wC  1bzdC  1bzeC  1tshC  2trhC  1ttaS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.99044, 0.13681, -0.01781), Vector: |
- Components
Components
| #1: Protein | Mass: 13853.457 Da / Num. of mol.: 2 / Mutation: VARIANT S77Y Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell line: HB101 / Plasmid: PCZ11 / Cell line (production host): HB101 / Production host: Homo sapiens (human) / Cell line: HB101 / Plasmid: PCZ11 / Cell line (production host): HB101 / Production host:  #2: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 52.5 % |
|---|---|
| Crystal grow | pH: 6.5 Details: PURIFIED PROTEIN (25MG/ML IN 100MM TRIS BUFFER, PH 7.5) WAS CRYSTALLIZED FROM 1.5M AMMONIUM SULFATE, 100MM CITRATE BUFFER, PH 5.5, AT ROOM TEMPERATURE., pH 6.5 PH range: 5.5-7.5 / Temp details: room temp |
-Data collection
| Diffraction | Mean temperature: 296 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 |
| Detector | Type: RIGAKU / Detector: IMAGE PLATE / Date: Dec 1, 1995 / Details: COLLIMATOR |
| Radiation | Monochromator: GRAPHITE(002) / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→64.9 Å / Num. obs: 17581 / % possible obs: 74.9 % / Observed criterion σ(I): 1 / Redundancy: 2.2 % / Biso Wilson estimate: 11.1 Å2 / Rmerge(I) obs: 0.107 / Rsym value: 0.091 / Net I/σ(I): 5 |
| Reflection shell | Resolution: 1.8→2 Å / Redundancy: 1.5 % / Rmerge(I) obs: 0.27 / Mean I/σ(I) obs: 1.8 / Rsym value: 0.22 / % possible all: 56 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1TTA Resolution: 2→5 Å / Rfactor Rfree error: 0.011 / Data cutoff high absF: 515.8 / Data cutoff low absF: 7 / Cross valid method: THROUGHOUT / σ(F): 2 Details: RESIDUES IN REGIONS WITH LOW ELECTRON DENSITY (RESIDUES 1 - 9 AT N-TERMINUS AND RESIDUES 124 - 127 AT C-TERMINUS) WERE REFINED WITH OCCUPANCIES SET TO 0.5.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 19.8 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→5 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2→2.08 Å / Rfactor Rfree error: 0.048 / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj





