[English] 日本語
 Yorodumi
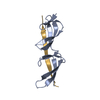
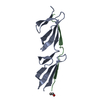
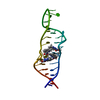
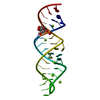
Yorodumi- PDB-2rkz: Crystal structure of the second and third fibronectin f1 modules ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2rkz | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the second and third fibronectin f1 modules in complex with a fragment of staphylococcus aureus fnbpa-1 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | CELL ADHESION / fibrronectin / 2F13F1 / beta zipper / staphylococcus aureus / Acute phase / Alternative splicing / Extracellular matrix / Glycoprotein / Heparin-binding / Phosphorylation / Pyrrolidone carboxylic acid / Secreted / Sulfation / Cell wall / Peptidoglycan-anchor / Virulence | |||||||||
| Function / homology |  Function and homology information Function and homology informationaggregation of unicellular organisms / negative regulation of monocyte activation / negative regulation of transforming growth factor beta production / Extracellular matrix organization / positive regulation of substrate-dependent cell migration, cell attachment to substrate / Fibronectin matrix formation / calcium-independent cell-matrix adhesion / neural crest cell migration involved in autonomic nervous system development / fibrinogen complex / peptide cross-linking ...aggregation of unicellular organisms / negative regulation of monocyte activation / negative regulation of transforming growth factor beta production / Extracellular matrix organization / positive regulation of substrate-dependent cell migration, cell attachment to substrate / Fibronectin matrix formation / calcium-independent cell-matrix adhesion / neural crest cell migration involved in autonomic nervous system development / fibrinogen complex / peptide cross-linking / integrin activation / fibrinogen binding / ALK mutants bind TKIs / cell-substrate junction assembly / proteoglycan binding / extracellular matrix structural constituent / MET activates PTK2 signaling / Molecules associated with elastic fibres / peptidase activator activity / Syndecan interactions / biological process involved in interaction with symbiont / p130Cas linkage to MAPK signaling for integrins / response to muscle activity / endoplasmic reticulum-Golgi intermediate compartment / endodermal cell differentiation / regulation of protein phosphorylation / GRB2:SOS provides linkage to MAPK signaling for Integrins / fibronectin binding / basement membrane / Non-integrin membrane-ECM interactions / ECM proteoglycans / Integrin cell surface interactions / endothelial cell migration / regulation of ERK1 and ERK2 cascade / positive regulation of axon extension / collagen binding / Degradation of the extracellular matrix / Integrin signaling / extracellular matrix / substrate adhesion-dependent cell spreading / platelet alpha granule lumen / Turbulent (oscillatory, disturbed) flow shear stress activates signaling by PIEZO1 and integrins in endothelial cells / cell-matrix adhesion / acute-phase response / Cell surface interactions at the vascular wall / integrin-mediated signaling pathway / Post-translational protein phosphorylation / wound healing / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / response to wounding / integrin binding / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / positive regulation of fibroblast proliferation / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / Signaling by BRAF and RAF1 fusions / Signaling by ALK fusions and activated point mutants / Platelet degranulation / nervous system development / GPER1 signaling / regulation of cell shape / heparin binding / : / heart development / protease binding / angiogenesis / Interleukin-4 and Interleukin-13 signaling / blood microparticle / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / cell adhesion / apical plasma membrane / receptor ligand activity / endoplasmic reticulum lumen / signaling receptor binding / positive regulation of cell population proliferation / positive regulation of gene expression / extracellular space / extracellular exosome / extracellular region / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | |||||||||
 Authors Authors | Bingham, R.J. | |||||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.Usa / Year: 2008 Journal: Proc.Natl.Acad.Sci.Usa / Year: 2008Title: Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains Authors: Bingham, R.J. / Meenan, N.A. / Schwarz-Linek, U. / Turkenburg, J.P. / Garman, E.F. / Potts, J.R. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2rkz.cif.gz 2rkz.cif.gz | 160 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2rkz.ent.gz pdb2rkz.ent.gz | 124.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2rkz.json.gz 2rkz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rk/2rkz https://data.pdbj.org/pub/pdb/validation_reports/rk/2rkz ftp://data.pdbj.org/pub/pdb/validation_reports/rk/2rkz ftp://data.pdbj.org/pub/pdb/validation_reports/rk/2rkz | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 10120.389 Da / Num. of mol.: 6 / Fragment: UNP residues 93-182 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: FN1 / Production host: Homo sapiens (human) / Gene: FN1 / Production host:  PICHIA PASTORIS (fungus) / Strain (production host): GS115 / References: UniProt: P02751 PICHIA PASTORIS (fungus) / Strain (production host): GS115 / References: UniProt: P02751#2: Protein/peptide | Mass: 2459.572 Da / Num. of mol.: 6 / Fragment: UNP residues 529-549 / Source method: obtained synthetically / Details: Peptide synthesis Source: (synth.)  Staphylococcus aureus (strain NCTC 8325) (bacteria) Staphylococcus aureus (strain NCTC 8325) (bacteria)References: UniProt: P14738 #3: Chemical | #4: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.08 Å3/Da / Density % sol: 60.05 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 0.8M SUCCINIC ACID, pH7.0, VAPOR DIFFUSION, SITTING DROP, temperature 291K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.9762 Å / Beamline: ID29 / Wavelength: 0.9762 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jul 25, 2006 Details: Liquid nitrogen cooled channel-cut silicon monochromator and a cylindrical grazing incidence mirror |
| Radiation | Monochromator: SI 111 CHANNEL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9762 Å / Relative weight: 1 |
| Reflection | Resolution: 2→26.038 Å / Num. all: 61539 / Num. obs: 58424 / % possible obs: 99.9 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 3.7 / Redundancy: 3.6 % / Biso Wilson estimate: 19.1 Å2 / Rmerge(I) obs: 0.1 / Rsym value: 0.1 / Net I/σ(I): 11.5 |
| Reflection shell | Resolution: 2→2.11 Å / Redundancy: 3.7 % / Rmerge(I) obs: 0.272 / Mean I/σ(I) obs: 4.2 / Num. measured all: 33090 / Num. unique all: 8970 / Rsym value: 0.272 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: A previous lower resolution structure Resolution: 2→25.58 Å / Cor.coef. Fo:Fc: 0.942 / Cor.coef. Fo:Fc free: 0.888 / SU B: 4.723 / SU ML: 0.132 / Isotropic thermal model: ISOTROPIC / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.166 / ESU R Free: 0.169 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 24.358 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→25.58 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2→2.052 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller




 PDBj
PDBj