[English] 日本語
 Yorodumi
Yorodumi- PDB-2qg4: Crystal structure of human UDP-glucose dehydrogenase product comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2qg4 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human UDP-glucose dehydrogenase product complex with UDP-glucuronate | ||||||
 Components Components | UDP-glucose 6-dehydrogenase | ||||||
 Keywords Keywords | OXIDOREDUCTASE / UDP-GLUCOSE 6-DEHYDROGENASE / HEXAMER / STRUCTURAL GENOMICS / Structural Genomics Consortium / SGC | ||||||
| Function / homology |  Function and homology information Function and homology informationFormation of the active cofactor, UDP-glucuronate / UDP-glucose 6-dehydrogenase activity / UDP-glucose 6-dehydrogenase / UDP-glucuronate biosynthetic process / glycosaminoglycan biosynthetic process / chondroitin sulfate proteoglycan biosynthetic process / heparan sulfate proteoglycan biosynthetic process / gastrulation with mouth forming second / protein hexamerization / neuron development ...Formation of the active cofactor, UDP-glucuronate / UDP-glucose 6-dehydrogenase activity / UDP-glucose 6-dehydrogenase / UDP-glucuronate biosynthetic process / glycosaminoglycan biosynthetic process / chondroitin sulfate proteoglycan biosynthetic process / heparan sulfate proteoglycan biosynthetic process / gastrulation with mouth forming second / protein hexamerization / neuron development / NAD binding / extracellular exosome / nucleoplasm / identical protein binding / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Kavanagh, K.L. / Guo, K. / Bunkoczi, G. / Savitsky, P. / Pilka, E. / Bhatia, C. / Niesen, F. / Smee, C. / Berridge, G. / von Delft, F. ...Kavanagh, K.L. / Guo, K. / Bunkoczi, G. / Savitsky, P. / Pilka, E. / Bhatia, C. / Niesen, F. / Smee, C. / Berridge, G. / von Delft, F. / Weigelt, J. / Arrowsmith, C.H. / Sundstrom, M. / Edwards, A. / Oppermann, U. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2011 Journal: J.Biol.Chem. / Year: 2011Title: Structure and mechanism of human UDP-glucose 6-dehydrogenase. Authors: Egger, S. / Chaikuad, A. / Kavanagh, K.L. / Oppermann, U. / Nidetzky, B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2qg4.cif.gz 2qg4.cif.gz | 790.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2qg4.ent.gz pdb2qg4.ent.gz | 641.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2qg4.json.gz 2qg4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qg/2qg4 https://data.pdbj.org/pub/pdb/validation_reports/qg/2qg4 ftp://data.pdbj.org/pub/pdb/validation_reports/qg/2qg4 ftp://data.pdbj.org/pub/pdb/validation_reports/qg/2qg4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2q3eSC  3itkC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Beg auth comp-ID: PHE / Beg label comp-ID: PHE / End auth comp-ID: VAL / End label comp-ID: VAL / Refine code: 5 / Auth seq-ID: 2 - 466 / Label seq-ID: 3 - 467
NCS ensembles :
|
- Components
Components
-Protein , 1 types, 8 molecules ABCDEFGH
| #1: Protein | Mass: 52083.418 Da / Num. of mol.: 8 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: UGDH / Plasmid: PBEN1-SGC / Species (production host): Escherichia coli / Production host: Homo sapiens (human) / Gene: UGDH / Plasmid: PBEN1-SGC / Species (production host): Escherichia coli / Production host:  |
|---|
-Non-polymers , 5 types, 3027 molecules 
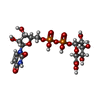







| #2: Chemical | ChemComp-NAD / #3: Chemical | ChemComp-UGA / #4: Chemical | ChemComp-CL / #5: Chemical | ChemComp-EDO / #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.06 Å3/Da / Density % sol: 59.75 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 20% PEG 3350, 10% Ethylene glycol, 0.2 M NaBr, 0.1 M Bis-tris-propane, pH 6.5, VAPOR DIFFUSION, SITTING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 1.033 Å / Beamline: X10SA / Wavelength: 1.033 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Apr 16, 2007 |
| Radiation | Monochromator: Si (111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.033 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→60 Å / Num. all: 288088 / Num. obs: 288088 / % possible obs: 100 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.9 % / Biso Wilson estimate: 17 Å2 / Rmerge(I) obs: 0.139 / Net I/σ(I): 7.6 |
| Reflection shell | Resolution: 2.1→2.21 Å / Redundancy: 3.8 % / Mean I/σ(I) obs: 1.8 / Num. unique all: 42048 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 2Q3E Resolution: 2.1→59.66 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.939 / SU B: 8.234 / SU ML: 0.117 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.177 / ESU R Free: 0.165 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 13.44 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→59.66 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Refine-ID: X-RAY DIFFRACTION
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





