| 登録情報 | データベース: PDB / ID: 2m1r
|
|---|
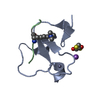
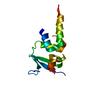
| タイトル | PHD domain of ING4 N214D mutant |
|---|
 要素 要素 | Inhibitor of growth protein 4 |
|---|
 キーワード キーワード | GENE REGULATION / ING4 / PHD / TRANSCRIPTION |
|---|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報
DNA replication-dependent chromatin disassembly / regulation of DNA biosynthetic process / negative regulation of growth / intermediate filament cytoskeleton / histone H3K4me3 reader activity / regulation of cell cycle G2/M phase transition / positive regulation of DNA damage response, signal transduction by p53 class mediator / protein acetylation / : / histone acetyltransferase complex ...DNA replication-dependent chromatin disassembly / regulation of DNA biosynthetic process / negative regulation of growth / intermediate filament cytoskeleton / histone H3K4me3 reader activity / regulation of cell cycle G2/M phase transition / positive regulation of DNA damage response, signal transduction by p53 class mediator / protein acetylation / : / histone acetyltransferase complex / regulation of cell growth / HATs acetylate histones / transcription coactivator activity / regulation of cell cycle / positive regulation of apoptotic process / chromatin remodeling / negative regulation of cell population proliferation / negative regulation of DNA-templated transcription / apoptotic process / regulation of DNA-templated transcription / zinc ion binding / nucleoplasm / nucleus / cytosol類似検索 - 分子機能 Inhibitor of growth protein, N-terminal histone-binding / Inhibitor of growth proteins N-terminal histone-binding / Inhibitor of growth proteins N-terminal histone-binding / ING family / Zinc/RING finger domain, C3HC4 (zinc finger) / Herpes Virus-1 / Zinc finger, PHD-type, conserved site / Zinc finger PHD-type signature. / Zinc finger PHD-type profile. / Zinc finger, PHD-finger ...Inhibitor of growth protein, N-terminal histone-binding / Inhibitor of growth proteins N-terminal histone-binding / Inhibitor of growth proteins N-terminal histone-binding / ING family / Zinc/RING finger domain, C3HC4 (zinc finger) / Herpes Virus-1 / Zinc finger, PHD-type, conserved site / Zinc finger PHD-type signature. / Zinc finger PHD-type profile. / Zinc finger, PHD-finger / Zinc finger, PHD-type / PHD zinc finger / Zinc finger, FYVE/PHD-type / Zinc finger, RING/FYVE/PHD-type / 2-Layer Sandwich / Alpha Beta類似検索 - ドメイン・相同性 |
|---|
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) |
|---|
| 手法 | 溶液NMR / torsion angle dynamics, molecular dynamics |
|---|
| Model details | lowest energy, model1 |
|---|
 データ登録者 データ登録者 | Blanco, F.J. |
|---|
 引用 引用 |  ジャーナル: Carcinogenesis / 年: 2010 ジャーナル: Carcinogenesis / 年: 2010
タイトル: Functional impact of cancer-associated mutations in the tumor suppressor protein ING4.
著者: Moreno, A. / Palacios, A. / Orgaz, J.L. / Jimenez, B. / Blanco, F.J. / Palmero, I. |
|---|
| 履歴 | | 登録 | 2012年12月5日 | 登録サイト: BMRB / 処理サイト: RCSB |
|---|
| 改定 1.0 | 2012年12月26日 | Provider: repository / タイプ: Initial release |
|---|
| 改定 1.1 | 2024年5月1日 | Group: Data collection / Database references / Derived calculations
カテゴリ: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_nmr_software / pdbx_nmr_spectrometer / pdbx_struct_conn_angle / struct_conn / struct_ref_seq_dif / struct_site
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_nmr_software.name / _pdbx_nmr_spectrometer.model / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id |
|---|
|
|---|
 データを開く
データを開く 基本情報
基本情報 要素
要素 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 引用
引用 ジャーナル: Carcinogenesis / 年: 2010
ジャーナル: Carcinogenesis / 年: 2010 構造の表示
構造の表示 Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク ダウンロード
ダウンロード 2m1r.cif.gz
2m1r.cif.gz PDBx/mmCIF形式
PDBx/mmCIF形式 pdb2m1r.ent.gz
pdb2m1r.ent.gz PDB形式
PDB形式 2m1r.json.gz
2m1r.json.gz PDBx/mmJSON形式
PDBx/mmJSON形式 その他のダウンロード
その他のダウンロード 2m1r_validation.pdf.gz
2m1r_validation.pdf.gz wwPDB検証レポート
wwPDB検証レポート 2m1r_full_validation.pdf.gz
2m1r_full_validation.pdf.gz 2m1r_validation.xml.gz
2m1r_validation.xml.gz 2m1r_validation.cif.gz
2m1r_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/m1/2m1r
https://data.pdbj.org/pub/pdb/validation_reports/m1/2m1r ftp://data.pdbj.org/pub/pdb/validation_reports/m1/2m1r
ftp://data.pdbj.org/pub/pdb/validation_reports/m1/2m1r リンク
リンク 集合体
集合体
 要素
要素 Homo sapiens (ヒト) / 遺伝子: ING4, My036 / Variant: isoform 1 / 発現宿主:
Homo sapiens (ヒト) / 遺伝子: ING4, My036 / Variant: isoform 1 / 発現宿主: 
 試料調製
試料調製 解析
解析 ムービー
ムービー コントローラー
コントローラー













 PDBj
PDBj

 HSQC
HSQC