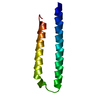
Entry Database : PDB / ID : 2lz3Title Solution NMR structure of transmembrane domain of amyloid precursor protein WT Amyloid beta A4 protein Keywords / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / Model type details minimized average Authors Chen, W. / Wang, C. #1: Journal : Protein Expr.Purif. / Year : 2012Title : Expression, purification, and reconstitution of the transmembrane domain of the human amyloid precursor protein for NMR studies.
Authors :
Chen, W. / Gamache, E. / Richardson, D. / Du, Z. / Wang, C. History Deposition Sep 23, 2012 Deposition site / Processing site Revision 1.0 Oct 2, 2013 Provider / Type Revision 1.1 Dec 18, 2013 Group Revision 1.2 Feb 5, 2014 Group Revision 1.3 Apr 27, 2016 Group Revision 1.4 May 1, 2024 Group / Database referencesCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / struct_ref_seq_dif Item / _database_2.pdbx_database_accession / _struct_ref_seq_dif.details
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) Authors
Authors Citation
Citation Journal: Nat Commun / Year: 2014
Journal: Nat Commun / Year: 2014 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2lz3.cif.gz
2lz3.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2lz3.ent.gz
pdb2lz3.ent.gz PDB format
PDB format 2lz3.json.gz
2lz3.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/lz/2lz3
https://data.pdbj.org/pub/pdb/validation_reports/lz/2lz3 ftp://data.pdbj.org/pub/pdb/validation_reports/lz/2lz3
ftp://data.pdbj.org/pub/pdb/validation_reports/lz/2lz3 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Plasmid: pETM41 / Production host:
Homo sapiens (human) / Plasmid: pETM41 / Production host: 
 Sample preparation
Sample preparation Processing
Processing Movie
Movie Controller
Controller











 PDBj
PDBj














 HSQC
HSQC