| Entry | Database: PDB / ID: 2ln3
|
|---|
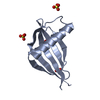
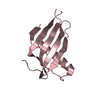
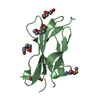
| Title | Solution NMR Structure of DE NOVO DESIGNED PROTEIN, IF3-like fold, Northeast Structural Genomics Consortium Target OR135 (CASD target) |
|---|
 Components Components | DE NOVO DESIGNED PROTEIN OR135 |
|---|
 Keywords Keywords | DE NOVO PROTEIN / Structural Genomics / NORTHEAST STRUCTURAL GENOMICS CONSORTIUM (NESG) / PSI-Biology / Protein Structure Initiative |
|---|
| Function / homology | Translation Initiation Factor IF3 - #140 / Translation Initiation Factor IF3 / 2-Layer Sandwich / Alpha Beta Function and homology information Function and homology information |
|---|
| Biological species | artificial gene (others) |
|---|
| Method | SOLUTION NMR / simulated annealing, molecular dynamics, distance geometry, torsion angle dynamics |
|---|
| Model details | lowest energy, model 1 |
|---|
 Authors Authors | Liu, G. / Koga, R. / Koga, N. / Xiao, R. / Lee, H. / Janjua, H. / Kohan, E. / Acton, T.B. / Everett, J.K. / Baker, D. ...Liu, G. / Koga, R. / Koga, N. / Xiao, R. / Lee, H. / Janjua, H. / Kohan, E. / Acton, T.B. / Everett, J.K. / Baker, D. / Montelione, G.T. / Northeast Structural Genomics Consortium (NESG) |
|---|
 Citation Citation |  Journal: Nature / Year: 2012 Journal: Nature / Year: 2012
Title: Principles for designing ideal protein structures.
Authors: Koga, N. / Tatsumi-Koga, R. / Liu, G. / Xiao, R. / Acton, T.B. / Montelione, G.T. / Baker, D. |
|---|
| History | | Deposition | Dec 15, 2011 | Deposition site: BMRB / Processing site: RCSB |
|---|
| Revision 1.0 | Feb 15, 2012 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Jun 13, 2012 | Group: Database references / Structure summary |
|---|
| Revision 1.2 | Oct 31, 2012 | Group: Database references |
|---|
| Revision 1.3 | Nov 7, 2012 | Group: Database references |
|---|
| Revision 1.4 | Jan 23, 2013 | Group: Database references |
|---|
| Revision 1.5 | Jun 14, 2023 | Group: Data collection / Database references / Other
Category: database_2 / pdbx_database_status ...database_2 / pdbx_database_status / pdbx_nmr_software / pdbx_nmr_spectrometer
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_nmr_data / _pdbx_nmr_software.name / _pdbx_nmr_spectrometer.model |
|---|
| Revision 1.6 | May 15, 2024 | Group: Data collection / Database references / Category: chem_comp_atom / chem_comp_bond / database_2 / Item: _database_2.pdbx_DOI |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Authors
Authors Citation
Citation Journal: Nature / Year: 2012
Journal: Nature / Year: 2012 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2ln3.cif.gz
2ln3.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2ln3.ent.gz
pdb2ln3.ent.gz PDB format
PDB format 2ln3.json.gz
2ln3.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ln/2ln3
https://data.pdbj.org/pub/pdb/validation_reports/ln/2ln3 ftp://data.pdbj.org/pub/pdb/validation_reports/ln/2ln3
ftp://data.pdbj.org/pub/pdb/validation_reports/ln/2ln3



 Links
Links Assembly
Assembly
 Components
Components
 Sample preparation
Sample preparation Processing
Processing Movie
Movie Controller
Controller









 PDBj
PDBj
 HSQC
HSQC