[English] 日本語
 Yorodumi
Yorodumi- PDB-2jxa: Mouse Latrophilin-1 GPCR Gal_lectin domain in complex with Rhamnose -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2jxa | ||||||
|---|---|---|---|---|---|---|---|
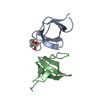
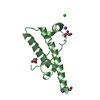
| Title | Mouse Latrophilin-1 GPCR Gal_lectin domain in complex with Rhamnose | ||||||
 Components Components | Latrophilin 1 | ||||||
 Keywords Keywords | CELL ADHESION / SIGNALING PROTEIN / Lectin / beta-sandwich / disulphide / glycosylated / L-Rhamnose / complex / G-protein coupled receptor / Membrane / Receptor / Transducer / Transmembrane | ||||||
| Function / homology |  Function and homology information Function and homology informationlatrotoxin receptor activity / positive regulation of synapse assembly / cell adhesion molecule binding / G protein-coupled receptor activity / presynaptic membrane / growth cone / carbohydrate binding / cell surface receptor signaling pathway / neuron projection / axon ...latrotoxin receptor activity / positive regulation of synapse assembly / cell adhesion molecule binding / G protein-coupled receptor activity / presynaptic membrane / growth cone / carbohydrate binding / cell surface receptor signaling pathway / neuron projection / axon / synapse / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | SOLUTION NMR / simulated annealing | ||||||
 Authors Authors | Vakonakis, I. / Campbell, I.D. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Solution structure and sugar-binding mechanism of mouse Latrophilin-1 RBL: a novel 7TM receptor-attached lectin-like domain. Authors: Vakonakis, I. / Langenhan, T. / Promel, S. / Russ, A. / Campbell, I.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2jxa.cif.gz 2jxa.cif.gz | 820.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2jxa.ent.gz pdb2jxa.ent.gz | 684.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2jxa.json.gz 2jxa.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2jxa_validation.pdf.gz 2jxa_validation.pdf.gz | 397.6 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2jxa_full_validation.pdf.gz 2jxa_full_validation.pdf.gz | 554.1 KB | Display | |
| Data in XML |  2jxa_validation.xml.gz 2jxa_validation.xml.gz | 54.6 KB | Display | |
| Data in CIF |  2jxa_validation.cif.gz 2jxa_validation.cif.gz | 86.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jx/2jxa https://data.pdbj.org/pub/pdb/validation_reports/jx/2jxa ftp://data.pdbj.org/pub/pdb/validation_reports/jx/2jxa ftp://data.pdbj.org/pub/pdb/validation_reports/jx/2jxa | HTTPS FTP |
-Related structure data
| Related structure data |  2jx9C C: citing same article ( |
|---|---|
| Similar structure data | |
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 11997.645 Da / Num. of mol.: 1 / Fragment: Gal_lectin Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Description: gene construct integrated in the organism's genome Gene: Lphn1 / Production host:  Pichia pastoris (fungus) / Strain (production host): X-33 / References: UniProt: Q5U4D5, UniProt: Q80TR1*PLUS Pichia pastoris (fungus) / Strain (production host): X-33 / References: UniProt: Q5U4D5, UniProt: Q80TR1*PLUS |
|---|---|
| #2: Sugar | ChemComp-NAG / |
| #3: Sugar | ChemComp-RAM / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj




 HSQC
HSQC