+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2gzv | ||||||
|---|---|---|---|---|---|---|---|
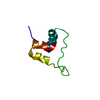
| Title | The cystal structure of the PDZ domain of human PICK1 | ||||||
 Components Components | PRKCA-binding protein | ||||||
 Keywords Keywords | SIGNALING PROTEIN / protein kinase C / PDZ domain / Structural Genomics / Structural Genomics Consortium / SGC | ||||||
| Function / homology |  Function and homology information Function and homology informationmembrane curvature sensor activity / glial cell development / neuronal ion channel clustering / cellular response to decreased oxygen levels / Trafficking of GluR2-containing AMPA receptors / Arp2/3 complex binding / negative regulation of Arp2/3 complex-mediated actin nucleation / regulation of Arp2/3 complex-mediated actin nucleation / monoamine transport / dendritic spine organization ...membrane curvature sensor activity / glial cell development / neuronal ion channel clustering / cellular response to decreased oxygen levels / Trafficking of GluR2-containing AMPA receptors / Arp2/3 complex binding / negative regulation of Arp2/3 complex-mediated actin nucleation / regulation of Arp2/3 complex-mediated actin nucleation / monoamine transport / dendritic spine organization / long-term synaptic depression / dendritic spine maintenance / receptor clustering / positive regulation of receptor internalization / cellular response to glucose starvation / regulation of insulin secretion / protein kinase C binding / trans-Golgi network membrane / Cell surface interactions at the vascular wall / intracellular protein transport / G protein-coupled receptor binding / phospholipid binding / synaptic vesicle / actin filament binding / endocytic vesicle membrane / presynaptic membrane / cytoskeleton / protein phosphorylation / neuron projection / postsynaptic density / signaling receptor binding / protein domain specific binding / synapse / perinuclear region of cytoplasm / Golgi apparatus / metal ion binding / identical protein binding / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.12 Å MOLECULAR REPLACEMENT / Resolution: 1.12 Å | ||||||
 Authors Authors | Debreczeni, J.E. / Elkins, J.M. / Yang, X. / Berridge, G. / Bray, J. / Colebrook, S. / Smee, C. / Savitsky, P. / Gileadi, O. / Turnbull, A. ...Debreczeni, J.E. / Elkins, J.M. / Yang, X. / Berridge, G. / Bray, J. / Colebrook, S. / Smee, C. / Savitsky, P. / Gileadi, O. / Turnbull, A. / von Delft, F. / Doyle, D.A. / Sundstrom, M. / Arrowsmith, C. / Weigelt, J. / Edwards, A. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 2007 Journal: Protein Sci. / Year: 2007Title: Structure of PICK1 and other PDZ domains obtained with the help of self-binding C-terminal extensions. Authors: Elkins, J.M. / Papagrigoriou, E. / Berridge, G. / Yang, X. / Phillips, C. / Gileadi, C. / Savitsky, P. / Doyle, D.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2gzv.cif.gz 2gzv.cif.gz | 55.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2gzv.ent.gz pdb2gzv.ent.gz | 39.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2gzv.json.gz 2gzv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gz/2gzv https://data.pdbj.org/pub/pdb/validation_reports/gz/2gzv ftp://data.pdbj.org/pub/pdb/validation_reports/gz/2gzv ftp://data.pdbj.org/pub/pdb/validation_reports/gz/2gzv | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2bygC  2fcfC  2fneSC  2he2C  2he4C  2i1nC  2iwnC  2iwoC  2iwpC  2iwqC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| 2 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
| ||||||||||||
| Details | The monomer present in the asymmetric unit is the biological unit. |
- Components
Components
| #1: Protein | Mass: 12427.161 Da / Num. of mol.: 1 / Fragment: PDZ domain, residues 19-105 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PICK1, PRKCABP / Plasmid: pNIC28-Bsa4 / Production host: Homo sapiens (human) / Gene: PICK1, PRKCABP / Plasmid: pNIC28-Bsa4 / Production host:  |
|---|---|
| #2: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.75 Å3/Da / Density % sol: 29.61 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 5.5 Details: 0.2 M Ammonium-acetate, 0.1 M Bis-Tris, 20% PEG3350, pH 5.5, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 0.97925 Å / Beamline: X10SA / Wavelength: 0.97925 Å |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: May 6, 2006 / Details: mirrors |
| Radiation | Monochromator: Si 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97925 Å / Relative weight: 1 |
| Reflection | Resolution: 1.12→29.3 Å / Num. all: 33346 / Num. obs: 32802 / % possible obs: 98.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Rmerge(I) obs: 0.0565 |
| Reflection shell | Resolution: 1.12→1.22 Å / Redundancy: 3.04 % / Rmerge(I) obs: 0.0126 / Mean I/σ(I) obs: 7.97 / Num. unique all: 7289 / % possible all: 94.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2FNE Resolution: 1.12→29.3 Å / Cor.coef. Fo:Fc: 0.973 / Cor.coef. Fo:Fc free: 0.97 / SU B: 0.795 / SU ML: 0.018 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.033 / ESU R Free: 0.032 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 9.872 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.12→29.3 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.118→1.147 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj

