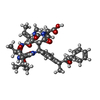
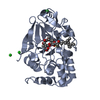
Entry Database : PDB / ID : 2bcdTitle X-ray crystal structure of Protein Phosphatase-1 with the marine toxin motuporin bound MOTUPORIN Serine/threonine protein phosphatase PP1-gamma catalytic subunit Keywords / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)marine sponge Thenonella swinhoie grey (invertebrata)Method / / / Resolution : 2.1 Å Authors Maynes, J.T. / Luu, H.A. / Cherney, M.M. / Andersen, R.J. / Williams, D. / Holmes, C.F. / James, M.N. Journal : J.Mol.Biol. / Year : 2006Title : Crystal Structures of Protein Phosphatase-1 Bound to Motuporin and Dihydromicrocystin-LA: Elucidation of the Mechanism of Enzyme Inhibition by Cyanobacterial Toxins.Authors : Maynes, J.T. / Luu, H.A. / Cherney, M.M. / Andersen, R.J. / Williams, D. / Holmes, C.F. / James, M.N. History Deposition Oct 19, 2005 Deposition site / Processing site Revision 1.0 Jan 17, 2006 Provider / Type Revision 1.1 May 1, 2008 Group Revision 1.2 Jul 13, 2011 Group Atomic model / Database references ... Atomic model / Database references / Derived calculations / Non-polymer description / Structure summary / Version format compliance Revision 1.3 Jul 27, 2011 Group Revision 1.4 Dec 12, 2012 Group Revision 1.5 Aug 23, 2023 Group Advisory / Data collection ... Advisory / Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / pdbx_struct_conn_angle / pdbx_struct_special_symmetry / pdbx_validate_polymer_linkage / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id Revision 2.0 Nov 15, 2023 Group / Data collection / Derived calculationsCategory atom_site / chem_comp_atom ... atom_site / chem_comp_atom / chem_comp_bond / pdbx_validate_main_chain_plane / pdbx_validate_peptide_omega / struct_conn Item _atom_site.auth_atom_id / _atom_site.label_atom_id ... _atom_site.auth_atom_id / _atom_site.label_atom_id / _chem_comp_atom.atom_id / _chem_comp_bond.atom_id_1 / _chem_comp_bond.atom_id_2 / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr2_label_atom_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) marine sponge Thenonella swinhoie grey (invertebrata)
marine sponge Thenonella swinhoie grey (invertebrata) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å
MOLECULAR REPLACEMENT / Resolution: 2.1 Å  Authors
Authors Citation
Citation Journal: J.Mol.Biol. / Year: 2006
Journal: J.Mol.Biol. / Year: 2006 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2bcd.cif.gz
2bcd.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2bcd.ent.gz
pdb2bcd.ent.gz PDB format
PDB format 2bcd.json.gz
2bcd.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/bc/2bcd
https://data.pdbj.org/pub/pdb/validation_reports/bc/2bcd ftp://data.pdbj.org/pub/pdb/validation_reports/bc/2bcd
ftp://data.pdbj.org/pub/pdb/validation_reports/bc/2bcd

 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: PPP1CC / Production host:
Homo sapiens (human) / Gene: PPP1CC / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ALS
ALS  / Beamline: 8.3.1 / Wavelength: 1 Å
/ Beamline: 8.3.1 / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller











 PDBj
PDBj