[English] 日本語
 Yorodumi
Yorodumi- PDB-2bc2: METALLO BETA-LACTAMASE II FROM BACILLUS CEREUS 569/H/9 AT PH 6.0,... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2bc2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | METALLO BETA-LACTAMASE II FROM BACILLUS CEREUS 569/H/9 AT PH 6.0, TRIGONAL CRYSTAL FORM | ||||||
 Components Components | METALLO BETA-LACTAMASE II | ||||||
 Keywords Keywords | HYDROLASE / BETA-LACTAMASE / ANTIBIOTIC / METALLOENZYME | ||||||
| Function / homology |  Function and homology information Function and homology informationantibiotic catabolic process / beta-lactamase activity / beta-lactamase / periplasmic space / response to antibiotic / zinc ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / DIFFERENCE FOURIER / Resolution: 1.7 Å SYNCHROTRON / DIFFERENCE FOURIER / Resolution: 1.7 Å | ||||||
 Authors Authors | Fabiane, S.M. / Sutton, B.J. | ||||||
 Citation Citation | #1:  Journal: Biochemistry / Year: 1998 Journal: Biochemistry / Year: 1998Title: Crystal Structure of the Zinc-Dependent Beta-Lactamase from Bacillus Cereus at 1.9 A Resolution: Binuclear Active Site with Features of a Mononuclear Enzyme Authors: Fabiane, S.M. / Sohi, M.K. / Wan, T. / Payne, D.J. / Bateson, J.H. / Mitchell, T. / Sutton, B.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2bc2.cif.gz 2bc2.cif.gz | 112.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2bc2.ent.gz pdb2bc2.ent.gz | 85.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2bc2.json.gz 2bc2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2bc2_validation.pdf.gz 2bc2_validation.pdf.gz | 438 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2bc2_full_validation.pdf.gz 2bc2_full_validation.pdf.gz | 440.7 KB | Display | |
| Data in XML |  2bc2_validation.xml.gz 2bc2_validation.xml.gz | 25.2 KB | Display | |
| Data in CIF |  2bc2_validation.cif.gz 2bc2_validation.cif.gz | 38.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bc/2bc2 https://data.pdbj.org/pub/pdb/validation_reports/bc/2bc2 ftp://data.pdbj.org/pub/pdb/validation_reports/bc/2bc2 ftp://data.pdbj.org/pub/pdb/validation_reports/bc/2bc2 | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 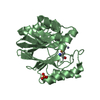
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (0.092376, 0.291462, 0.952112), Vector: |
- Components
Components
| #1: Protein | Mass: 25027.531 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #2: Chemical | #3: Chemical | ChemComp-SO4 / #4: Water | ChemComp-HOH / | Compound details | RESIDUES 1 - 4 WERE NOT PRESENT IN CRYSTALLIS | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.33 Å3/Da / Density % sol: 39 % |
|---|---|
| Crystal grow | pH: 6 / Details: pH 6.0 |
| Crystal | *PLUS |
| Crystal grow | *PLUS Method: otherDetails: This particular structure is not described in this paper. |
-Data collection
| Diffraction | Mean temperature: 153 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X12C / Wavelength: 1.1 / Beamline: X12C / Wavelength: 1.1 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE AREA DETECTOR / Date: Aug 1, 1996 / Details: MIRROR |
| Radiation | Monochromator: PAIR OF SINGLE FLAT CRYSTALS / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.7→50 Å / Num. obs: 52908 / % possible obs: 97.7 % / Observed criterion σ(I): 4 / Redundancy: 3.1 % / Biso Wilson estimate: 21.8 Å2 / Rsym value: 0.06 / Net I/σ(I): 11 |
| Reflection shell | Resolution: 1.68→1.72 Å / Redundancy: 2 % / Mean I/σ(I) obs: 2.2 / Rsym value: 0.309 / % possible all: 86 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: DIFFERENCE FOURIER / Resolution: 1.7→8 Å / Rfactor Rfree error: 0.0036 / Data cutoff high absF: 1000000 / Data cutoff low absF: 0.1 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / Details: NO NCS RESTRAINTS WERE USED.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 23.32 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.7→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.7→1.72 Å / Rfactor Rfree error: 0.021 / Total num. of bins used: 30
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.2 / Rfactor Rwork: 0.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






